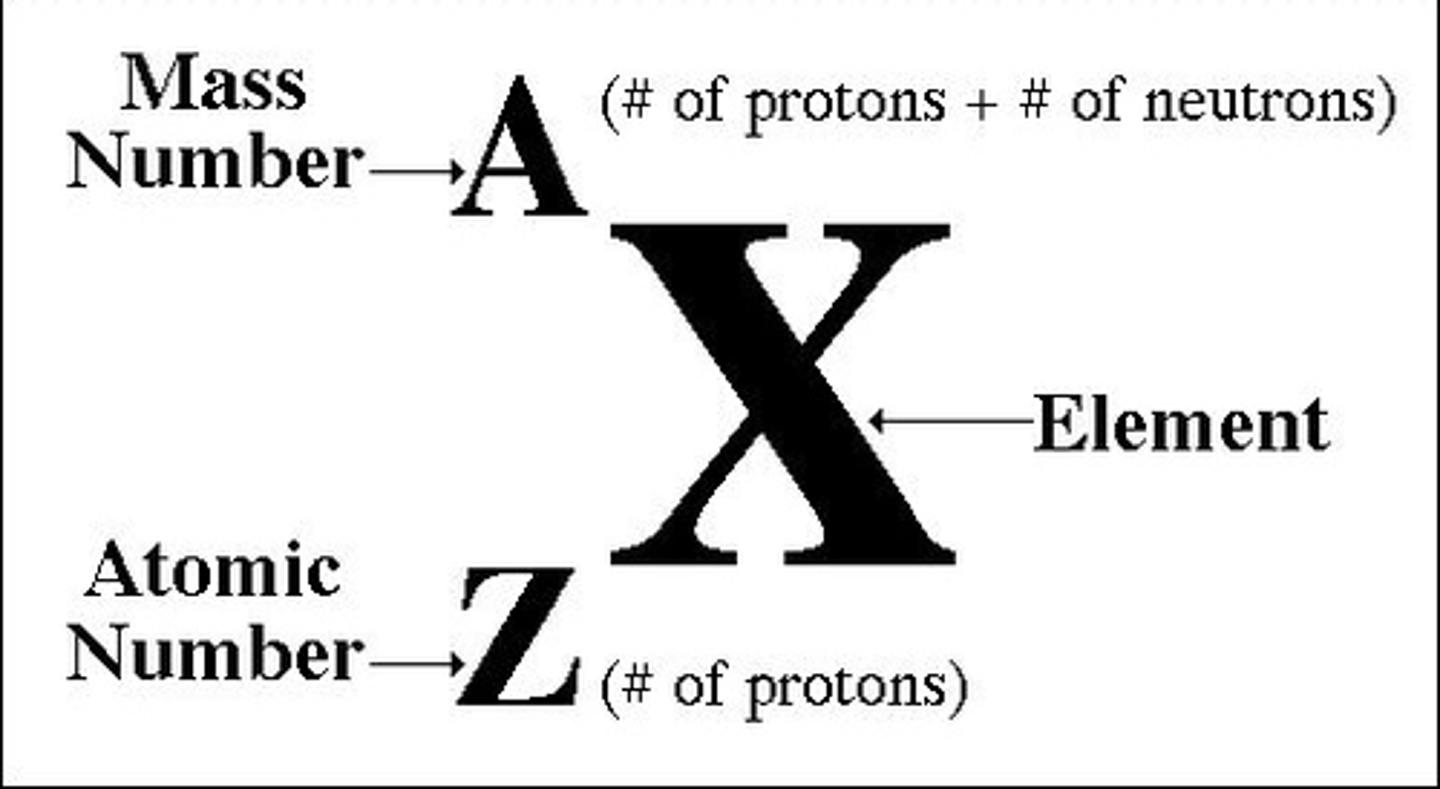SCH3U - Unit One
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
john dalton atomic theory
law of constant composition and law of conservation of mass

law of constant composition
all samples of that compound will be made up of the same elements in the same ratio
law of conservation of mass
matter cannot be created nor destroyed

thomson discovery
electrons can be emitted by very hot materials
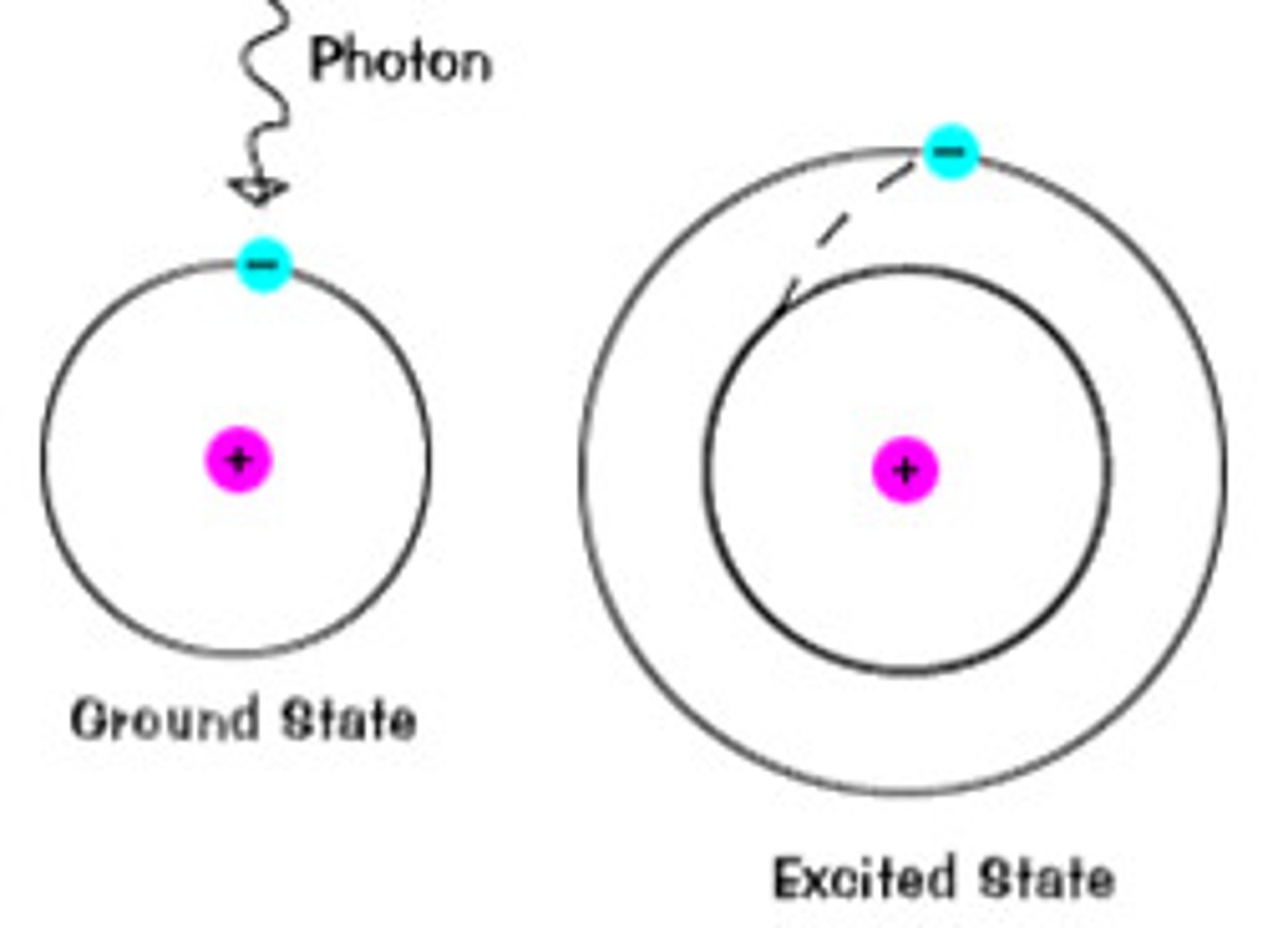
niels bohr findings
electrons in an atom can possess only discrete energy levels
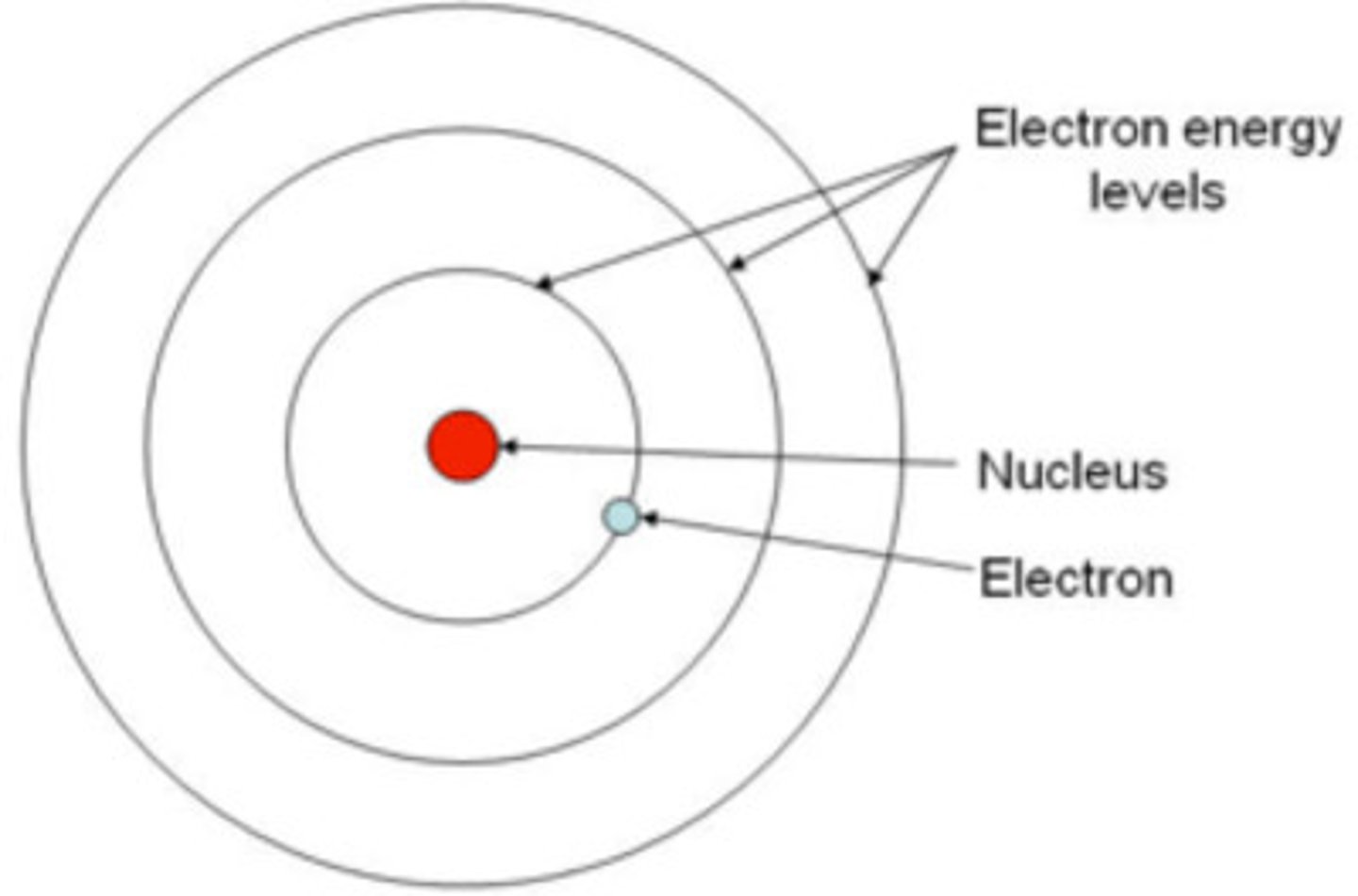
significance of bohr's findings
an electron can absorb energy and jump to a higher energy level, when it drops back to ground state, it emits excess energy as photons
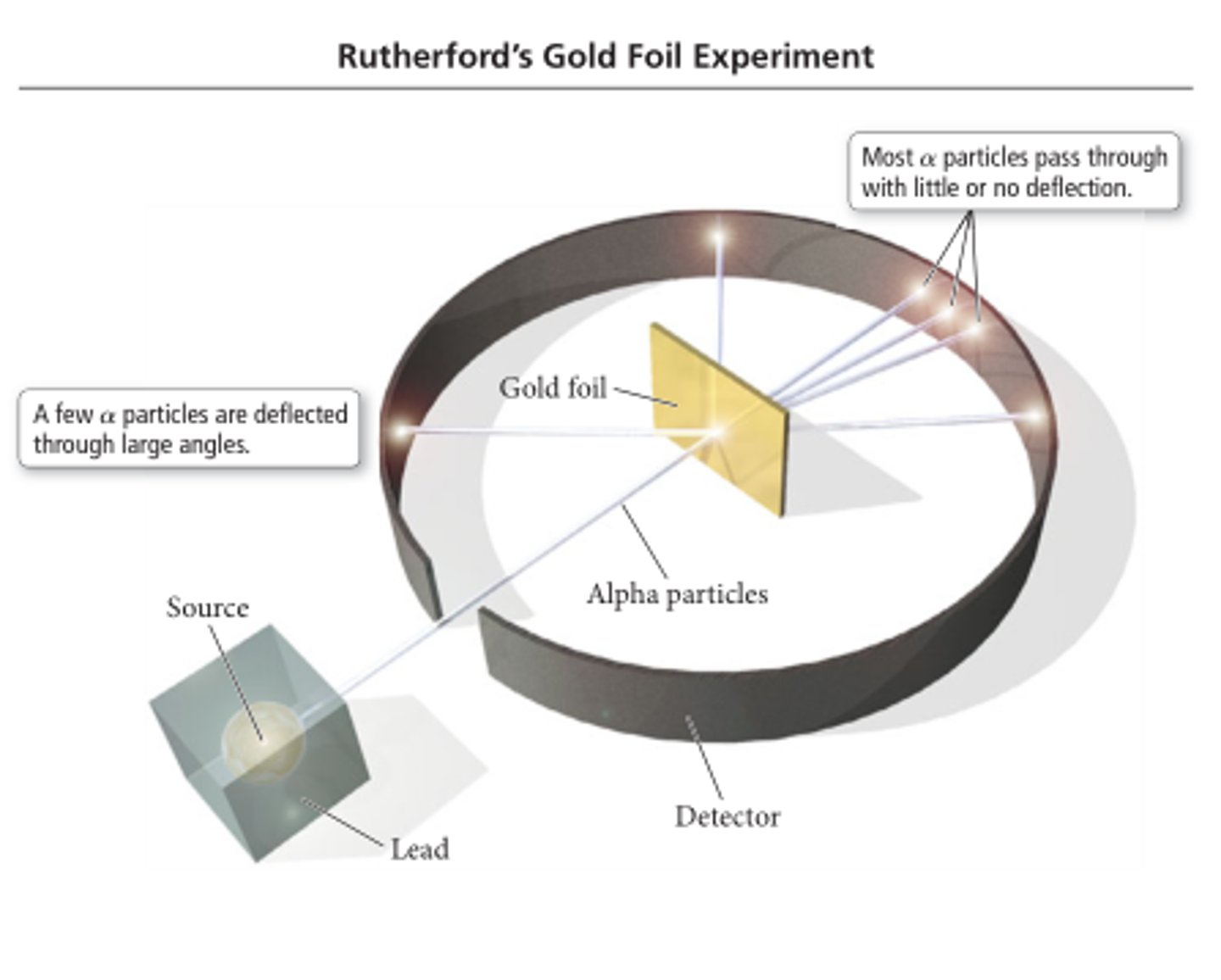
gold foil experiment
done by rutherford in which he shot alpha particles at gold foil, some particles passed straight through whilst others deflected at huge angles
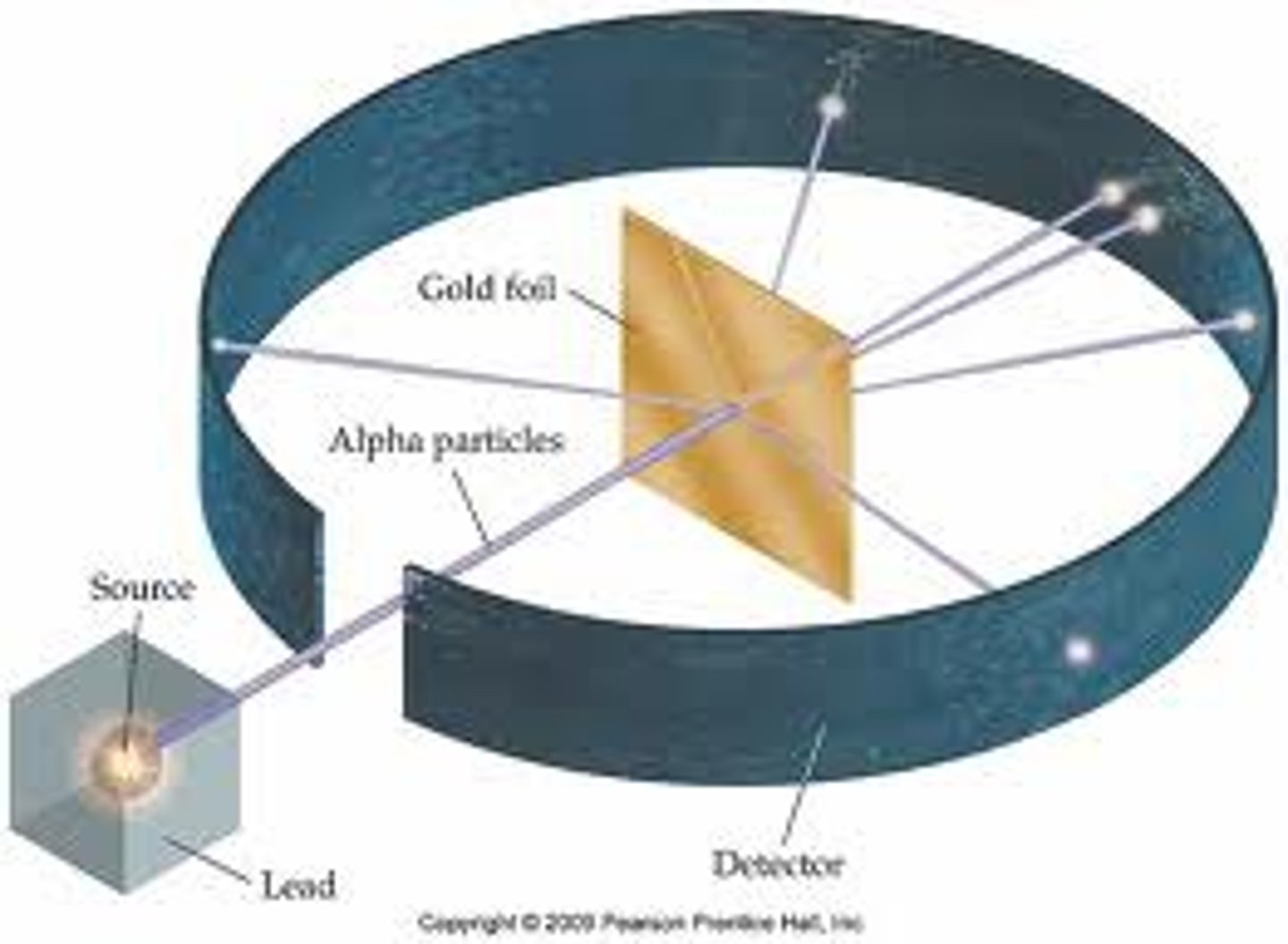
significance of gold foil experiment
showed that atoms were mostly made up of open space and had a positively charged nucleus, it showed this because the positively charged alpha particles repelled off of the gold due to the like charges
excited state
when an electron absorbs energy and moves to a higher energy level
photon
a particle of light
energy levels law
the greater the distance of an energy level from the nucleus, the greater energy required for an electron to travel
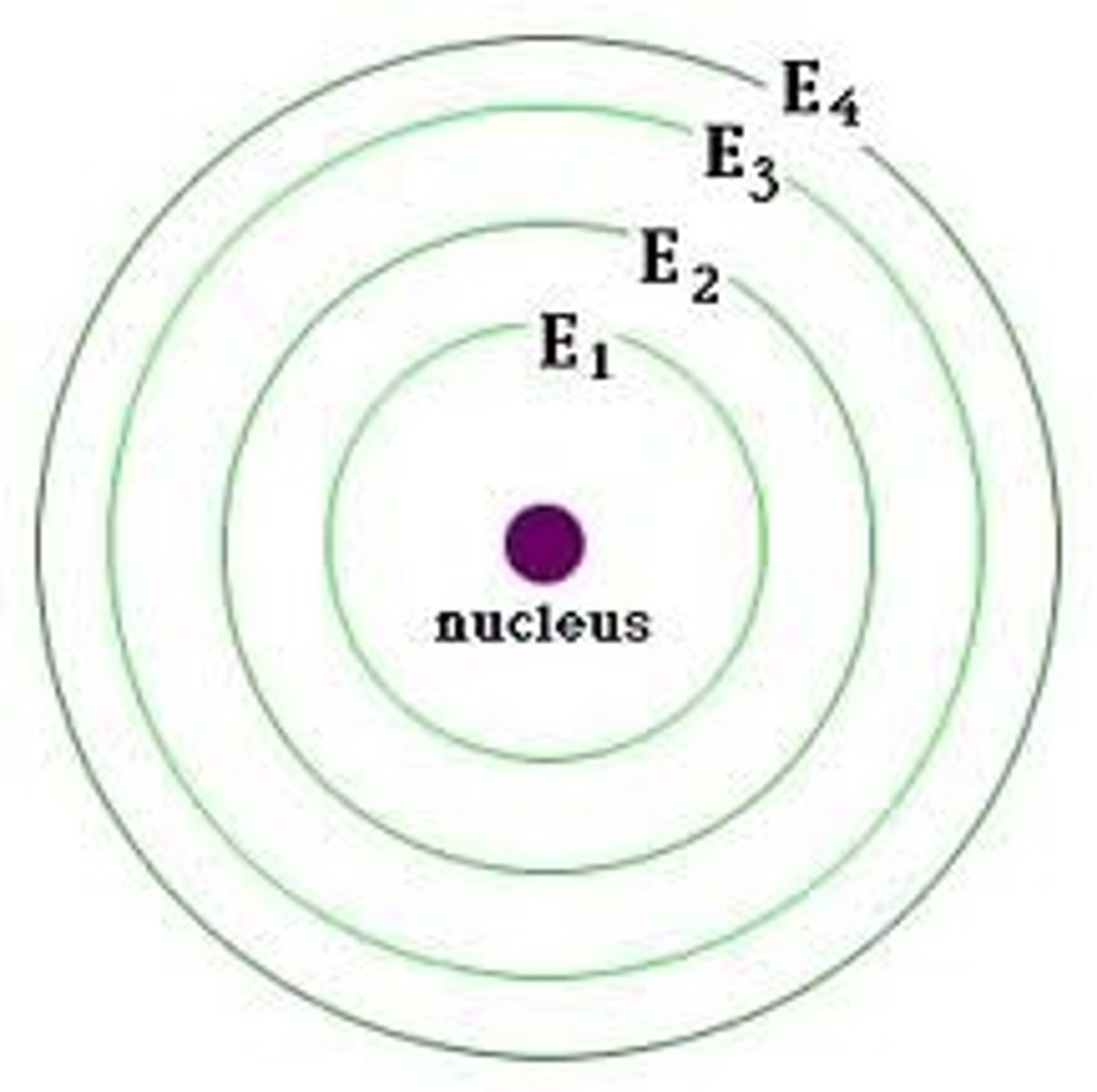
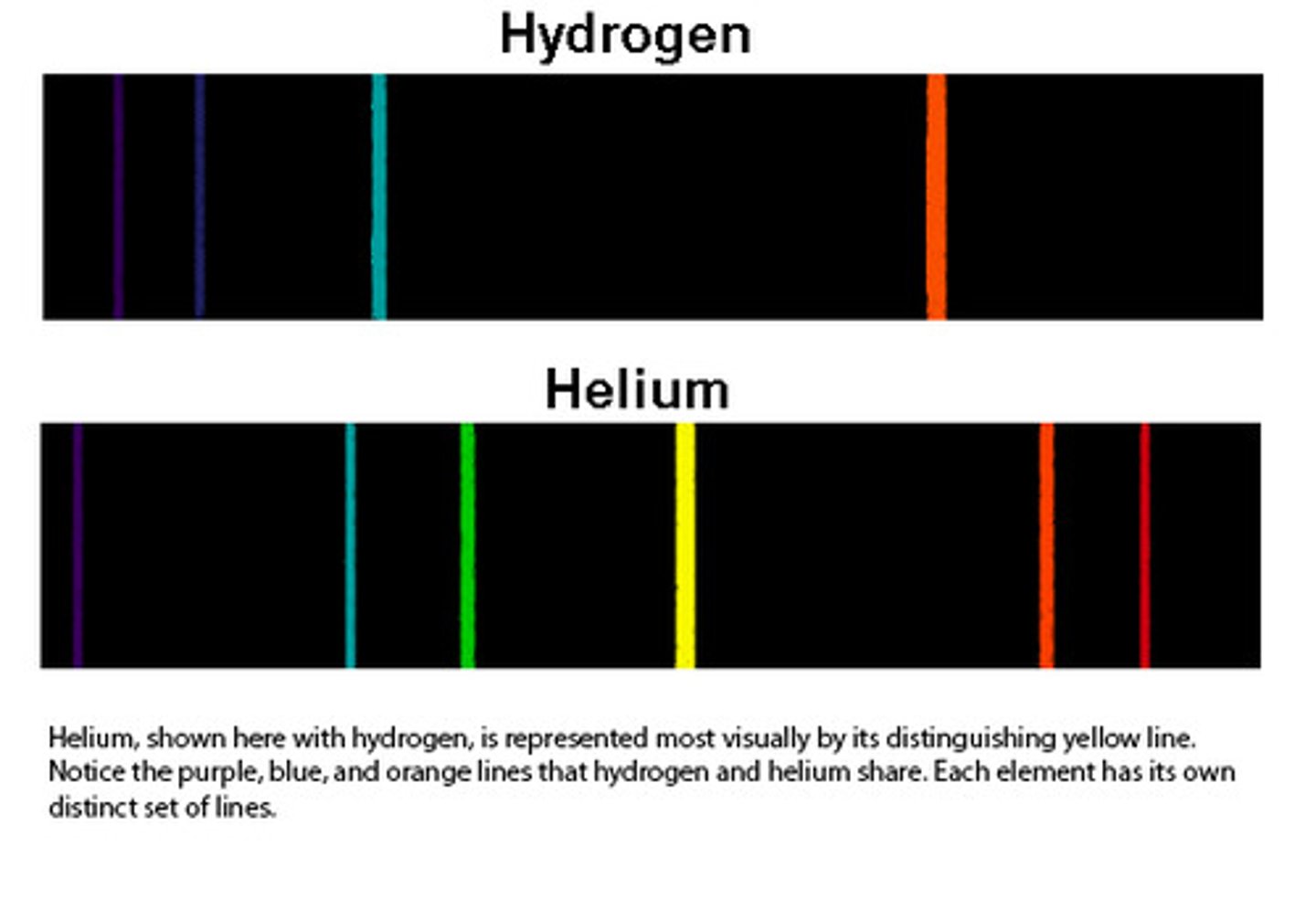
atomic line spectra
the photons emitted will produce different colours and types of electromagnetic radiation depending on the element, an element's line spectrum
flame test
testing different cations to see the color emitted from the flame, metal part of compound expresses certain color as the flame provides energy

atomic number
represents the amount of protons and electrons in a given element

mass number
the sum of all particles in the nucleus, typically rounded

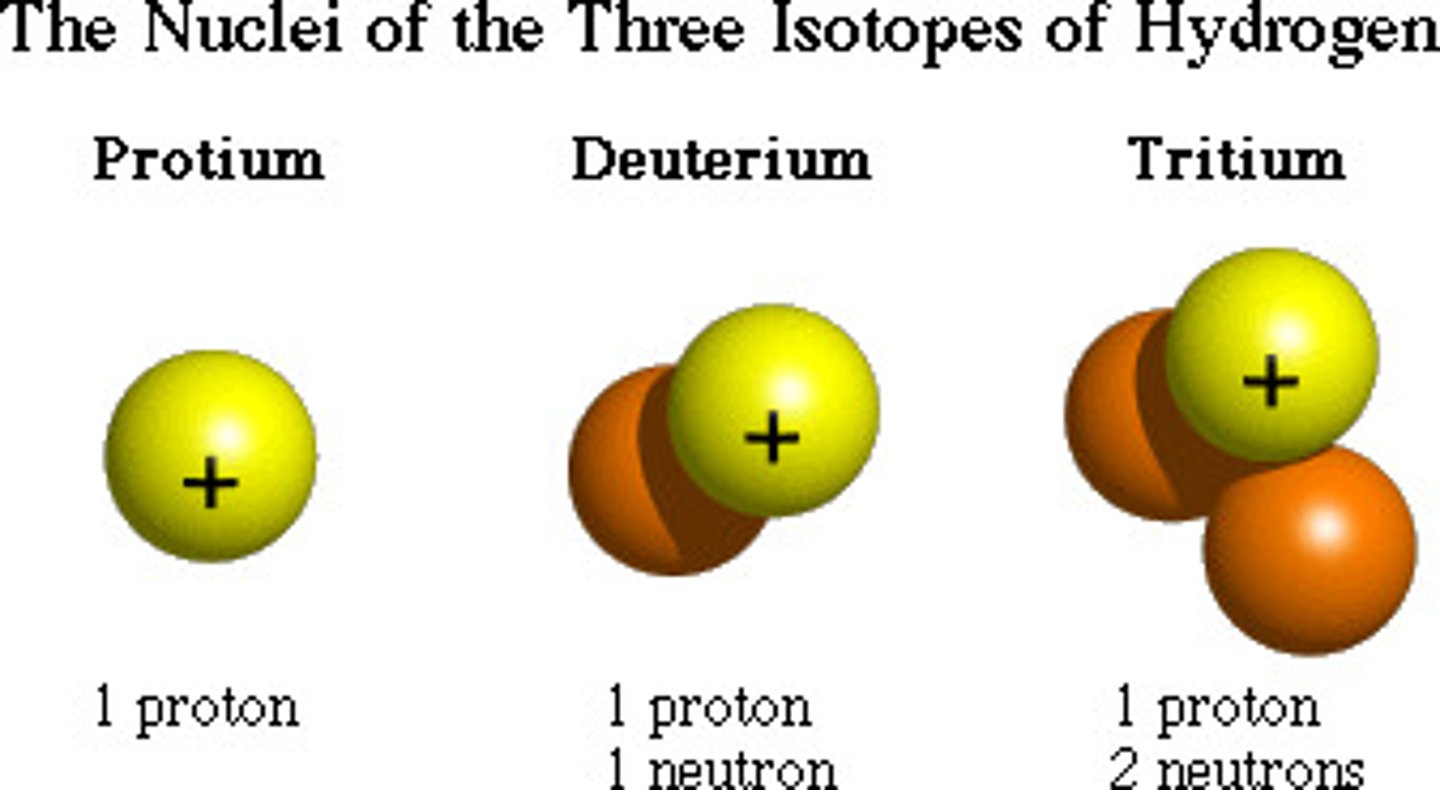
isotopes
different forms of an element have the same atomic number, meaning the exact same amount of protons, but differ in the amount of neutrons meaning different weight
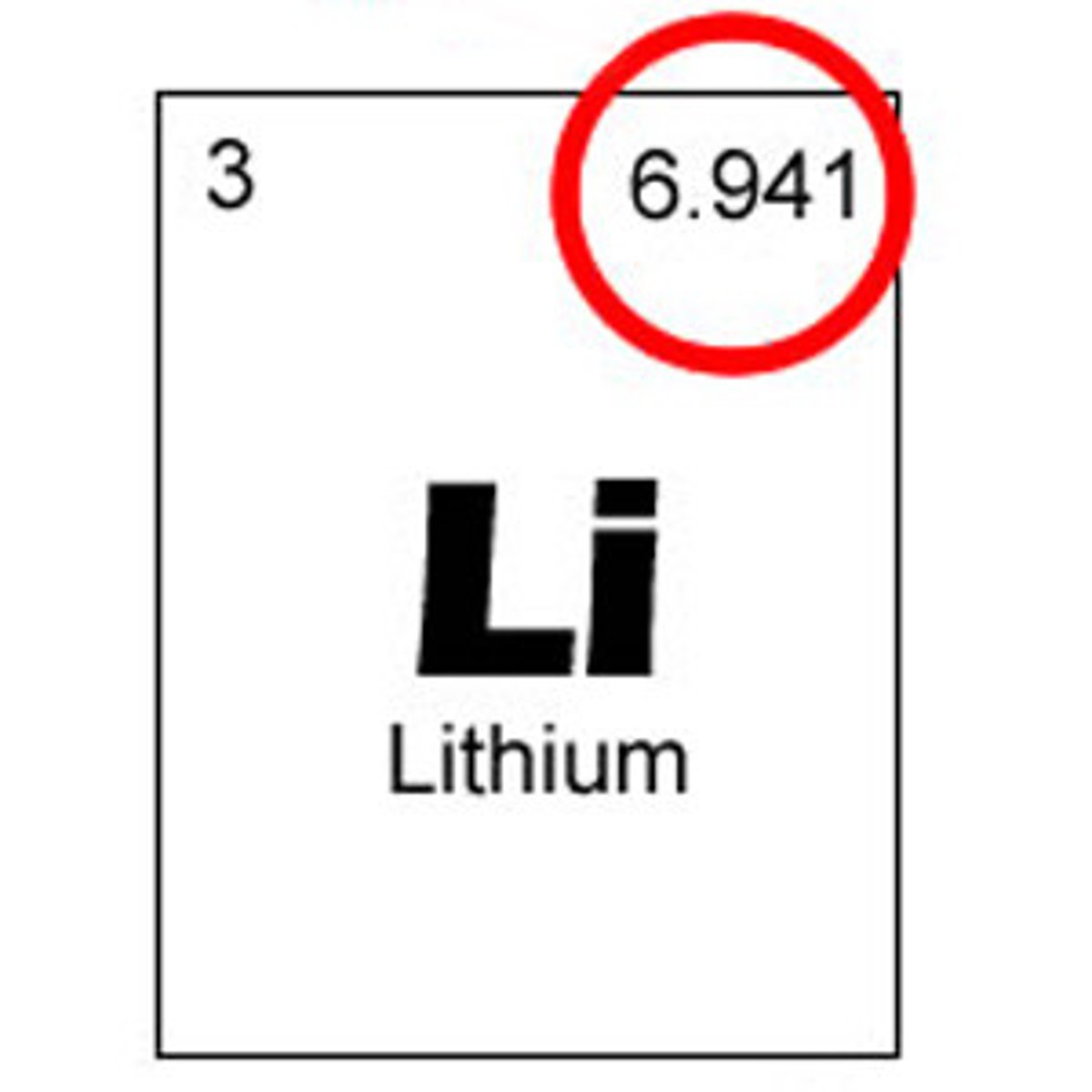
mass of an atom
expressed by unified atomic mass unit (u) the mass shown for an element on the periodic table is the average of all atomic masses of the element's different isotopes
isotopic abundance
the percentage of a given isotope in a sample of an element, out of 100 samples of a given element essentially how many will be of one given mass, and how many would be of the other
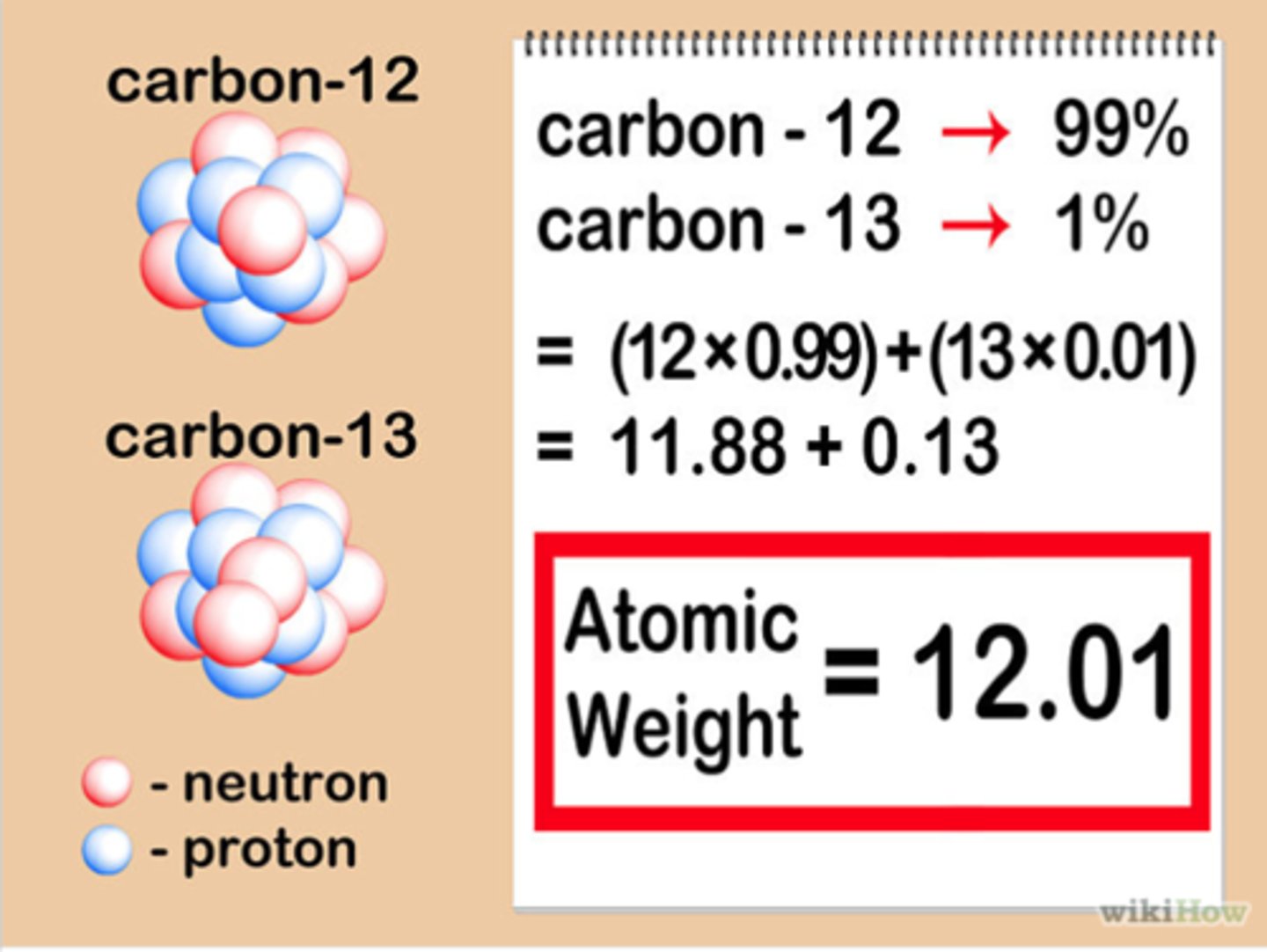
isotopic abundance formula
atomic mass = (% abundance of isotope one x mass of isotope one) + (% abundance of isotope 2 x mass of isotope 2) ... and so on if more isotopes are present
radioisotopes
elements may have isotopes that are unstable, that emit radiation posing either harmless or very dangerous

metals/non-metals on periodic table
to the left and right of the staircase
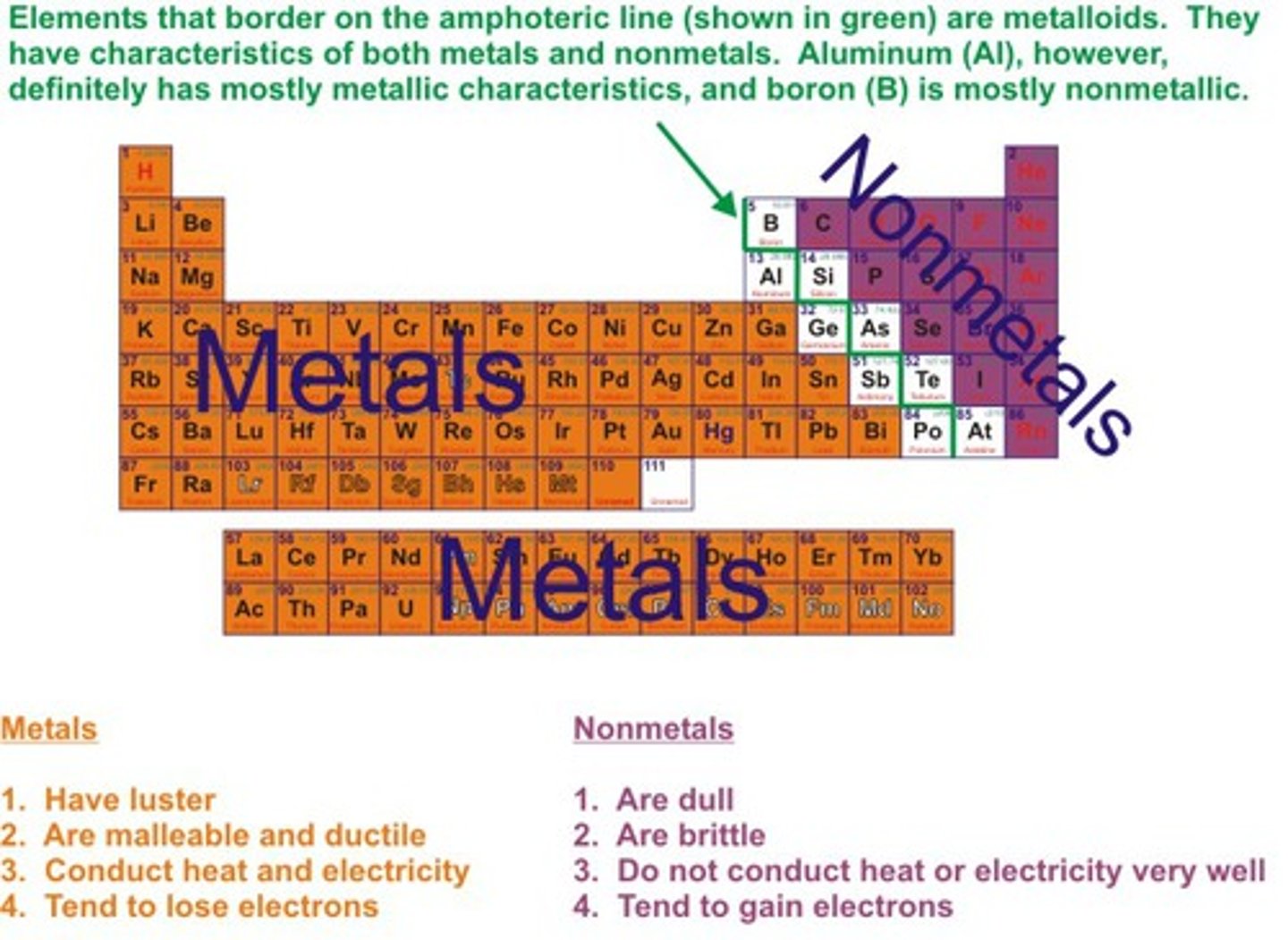
metalloids
elements that fall between metals and non-metals, thus forming the staircase
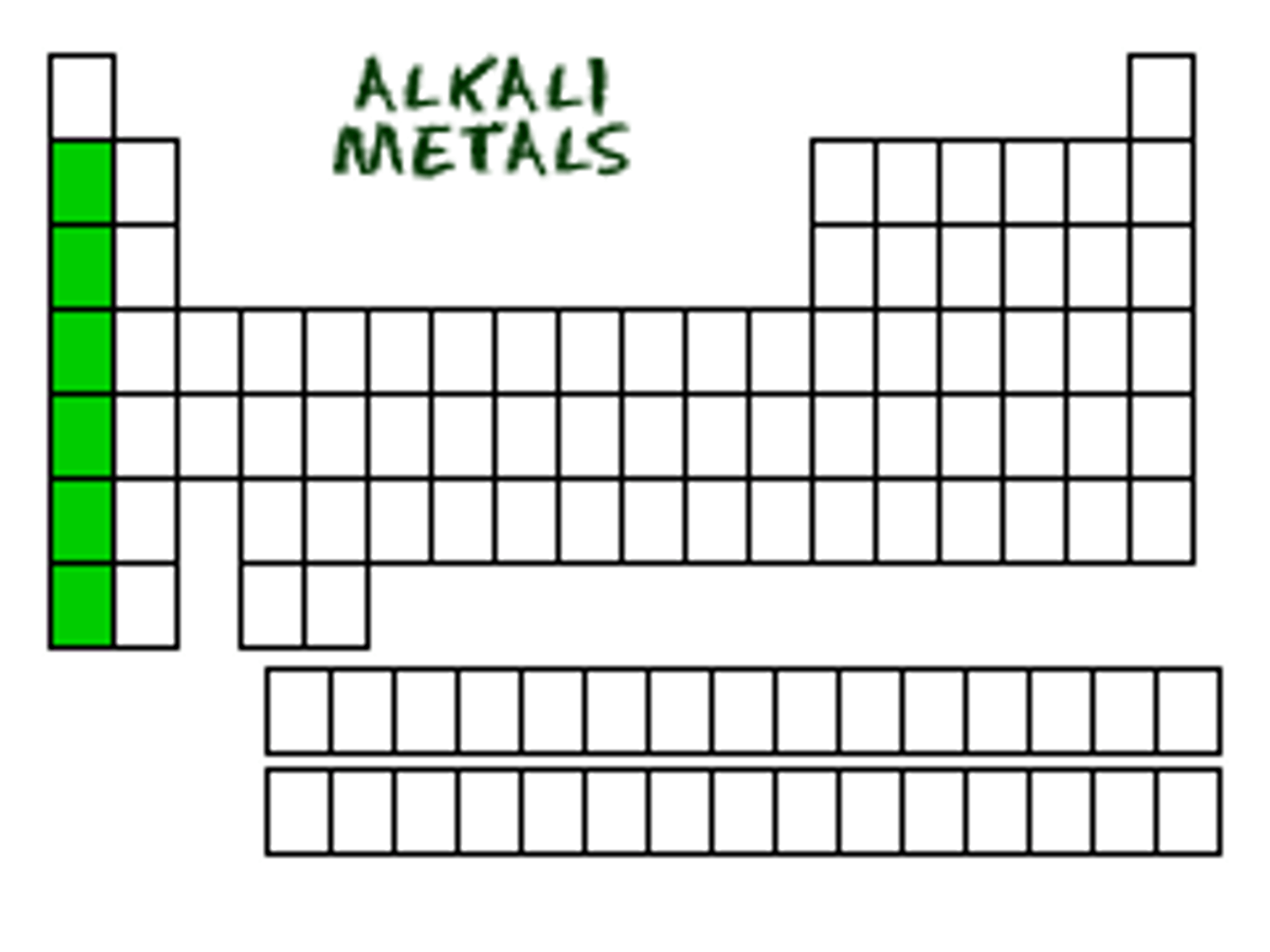
alkali metals
group one elements, extremely reactive
alkaline earth metals
group two elements, slightly reactive
halogens
group seventeen elements, low melting/boiling point
noble gases
group eighteen elements, cannot form bonds as they have stable valence shells
reactivity
an element's ability or tendency to form bonds at a certain speed/with certain amount of energy

reactivity trend
increases when traveling down and to the left in metals, increase when traveling up and to the right in non-metals
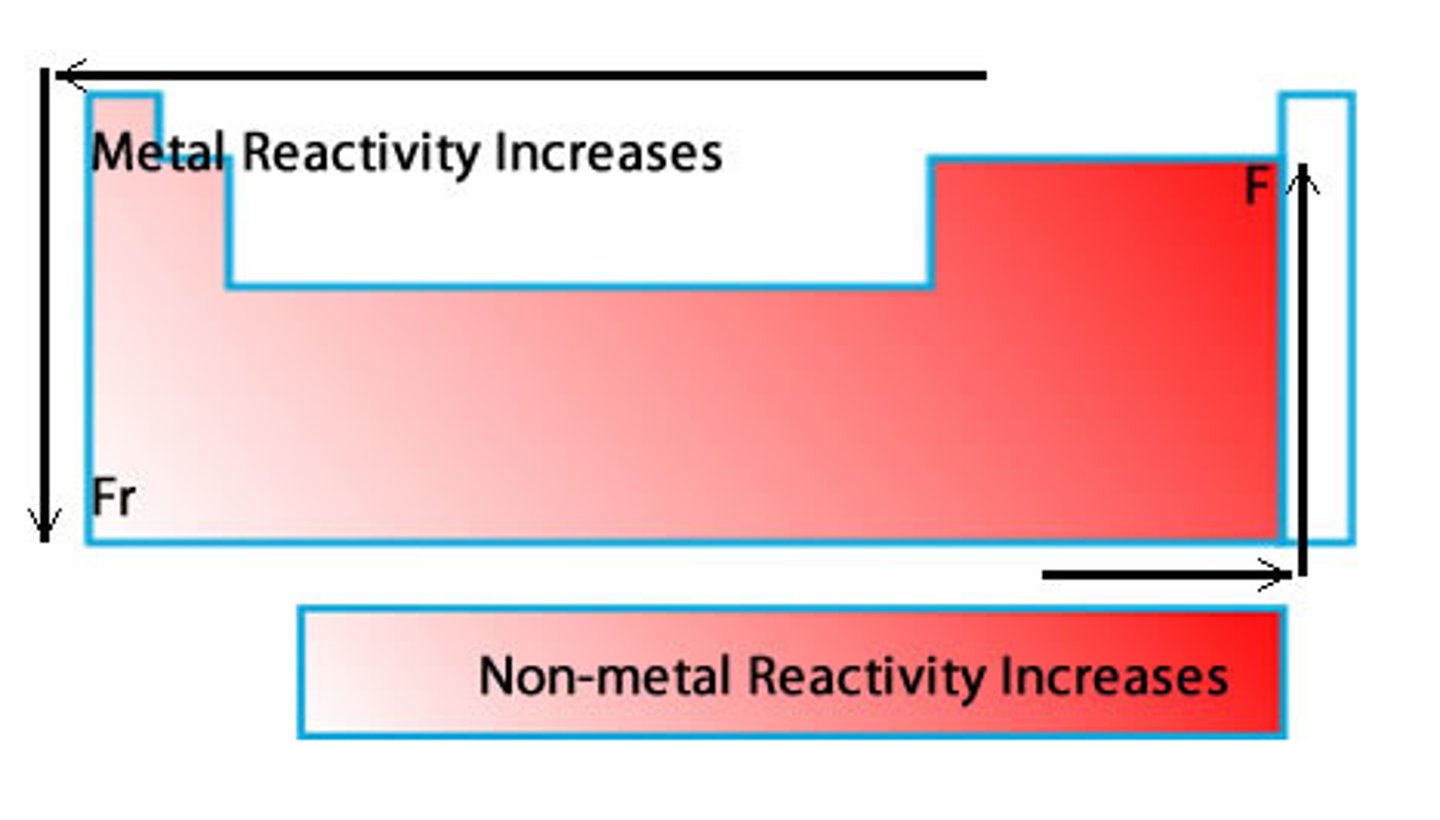
reason of reactivity trend (groups)
when traveling down, more shells are added which increases the electron shielding, thus reducing the amount of ionization energy required
atomic radius
the size of an atom/element
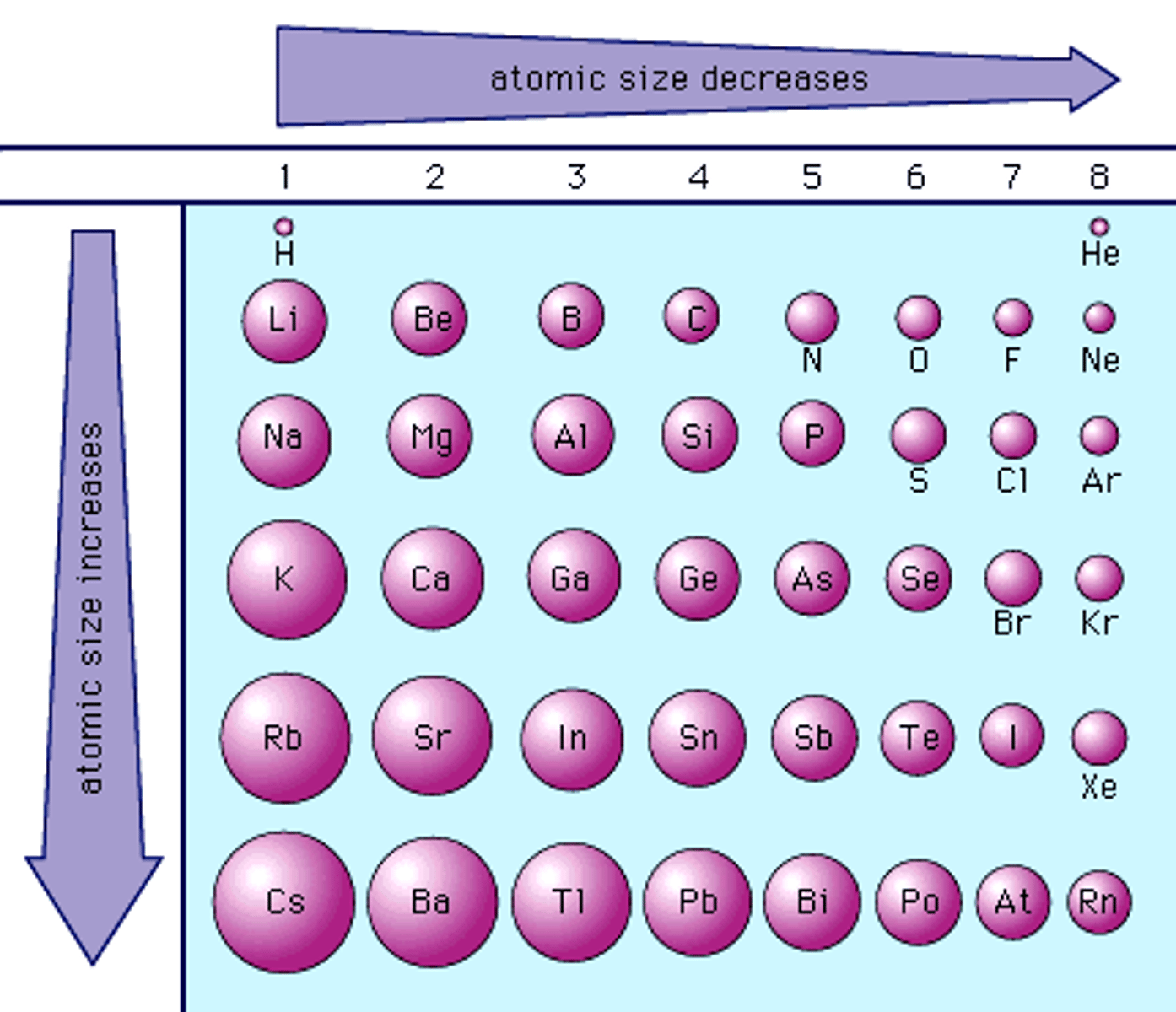
atomic radius trend
atomic radius increases when moving down, and decreases going left to right
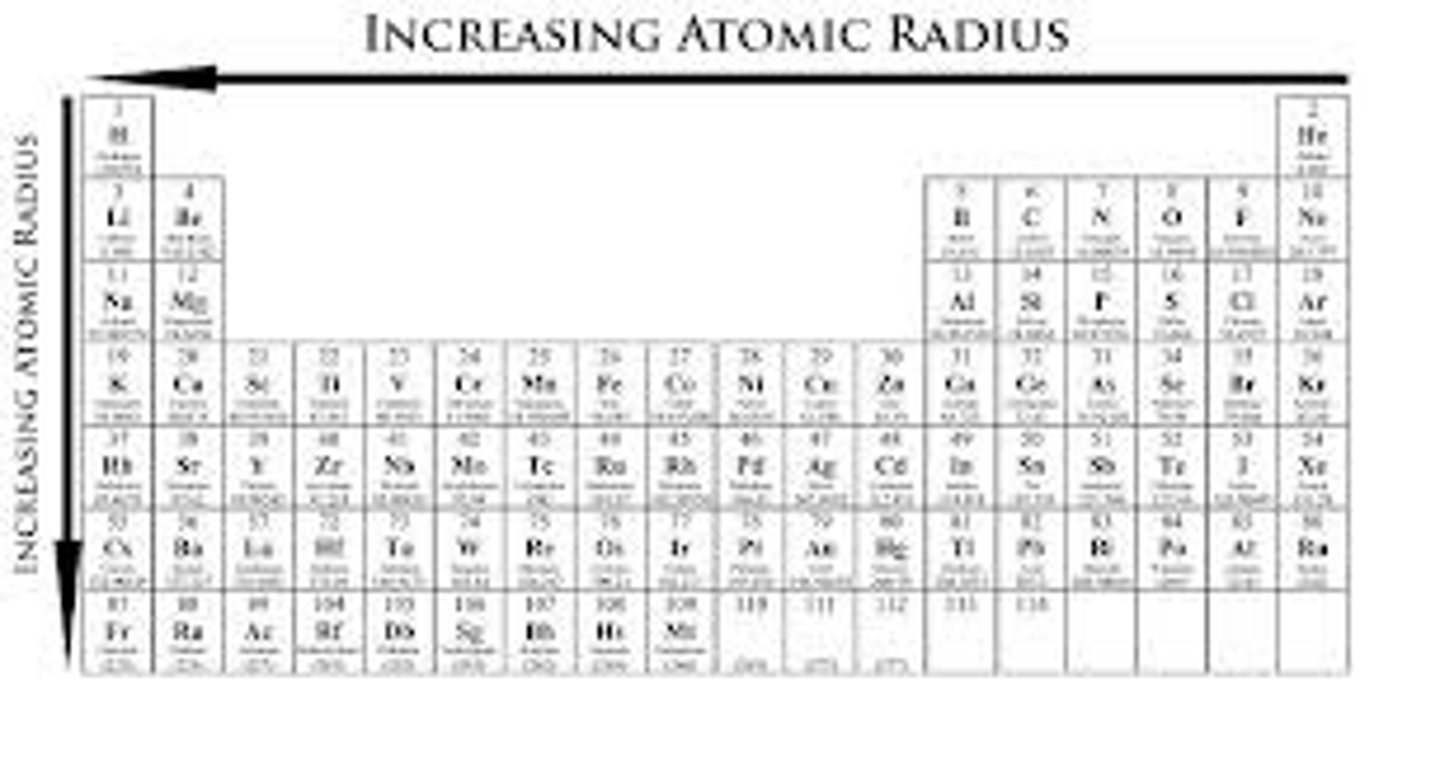
reason of atomic radius trend (groups)
when moving down a group, more electron shells are added thus increasing the electron shielding, this reduces the effective nuclear charge caused by the protons, limiting the pull
reason of atomic radius trend (periods)
when moving left to right in a period, the number of protons increase which increases the effective nuclear charge of the atom, thus causing a tighter pull.
ionic radius
the size of an atom when charged either positively or negatively
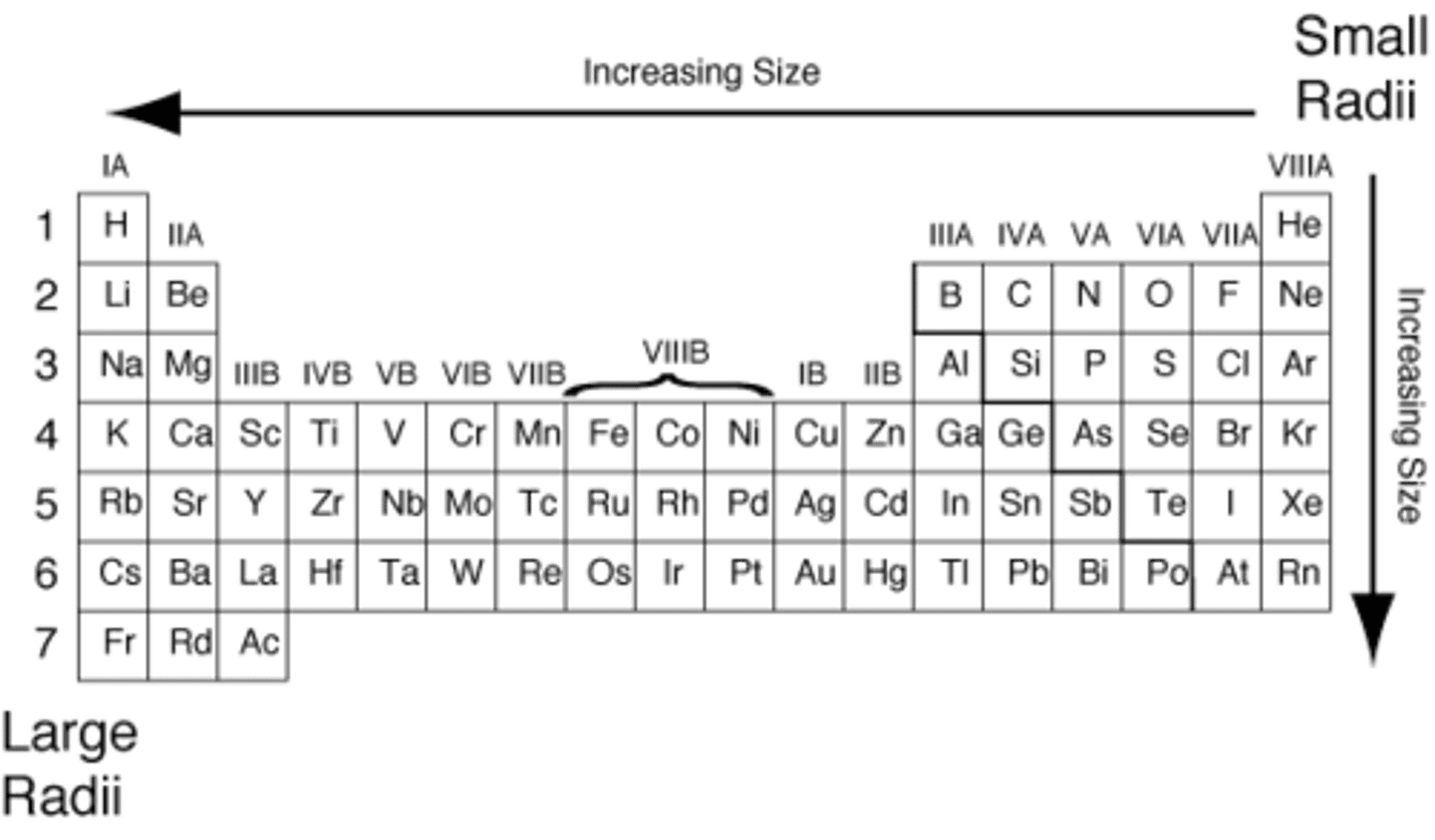
ionic radius trend (metals)
the ion will cause it to shrink (electrons removed)
ionic radius trend (non-metals)
the ion will cause it to grow (electrons added)
ionization energy
the amount of energy required to remove an electron from an atom, needed ionization energy increases for each electron removed --> 3, 2, 1 until none are left thus stable
ionization energy trend
increases when moving up a group and when moving to the right (more energy needed)
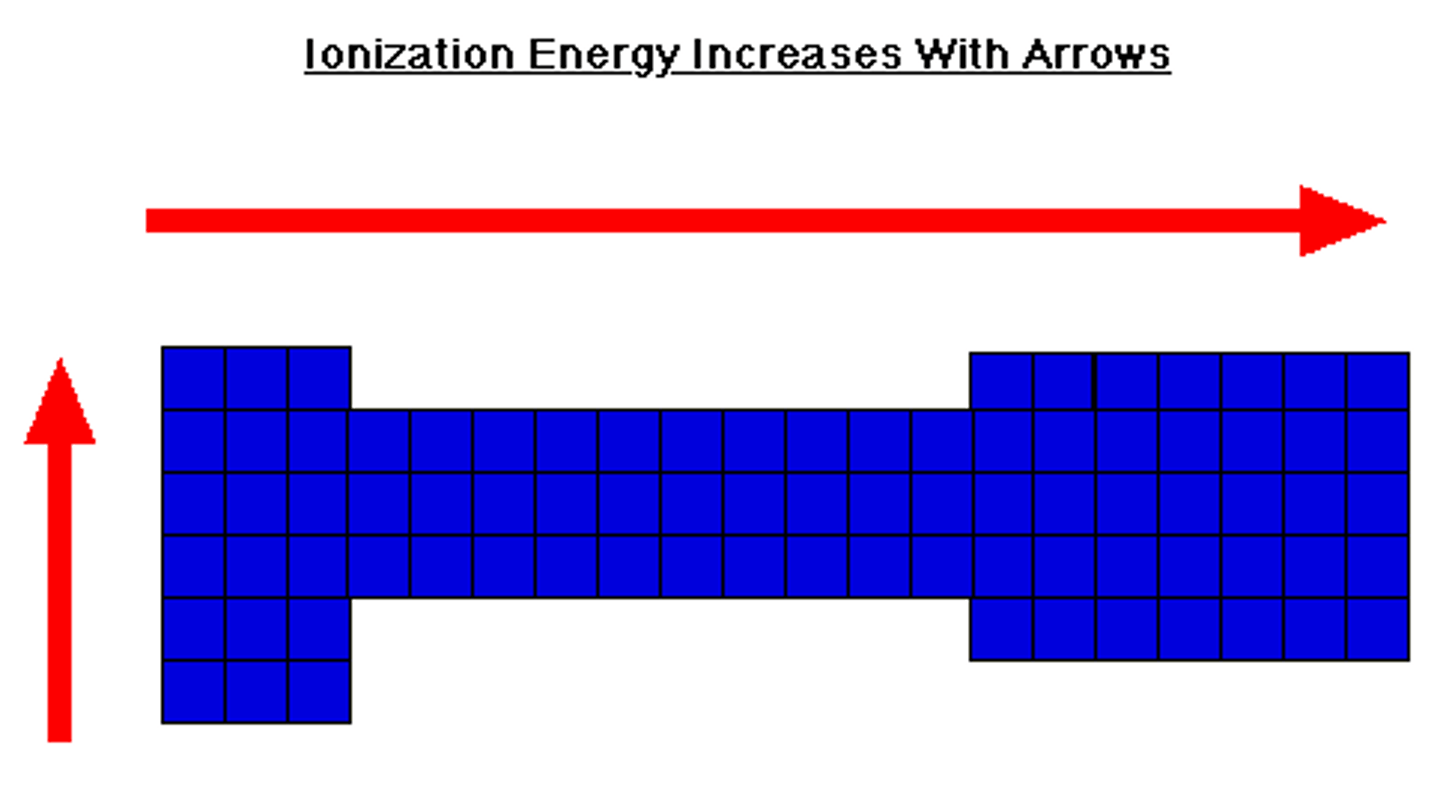
reason of ionization energy trend (groups)
when moving up a group, there are fewer shells which means less electron shielding, which causes the effective nuclear charge to be stronger on the valence electrons thus requiring more energy to remove it
reason of ionization energy trend (periods)
increases left to right because there are more valence electrons needed to be removed in order to be stable and also more protons causing a stronger pull against, ionization energy
electron affinity
the amount of energy released or absorbed when an electron is added
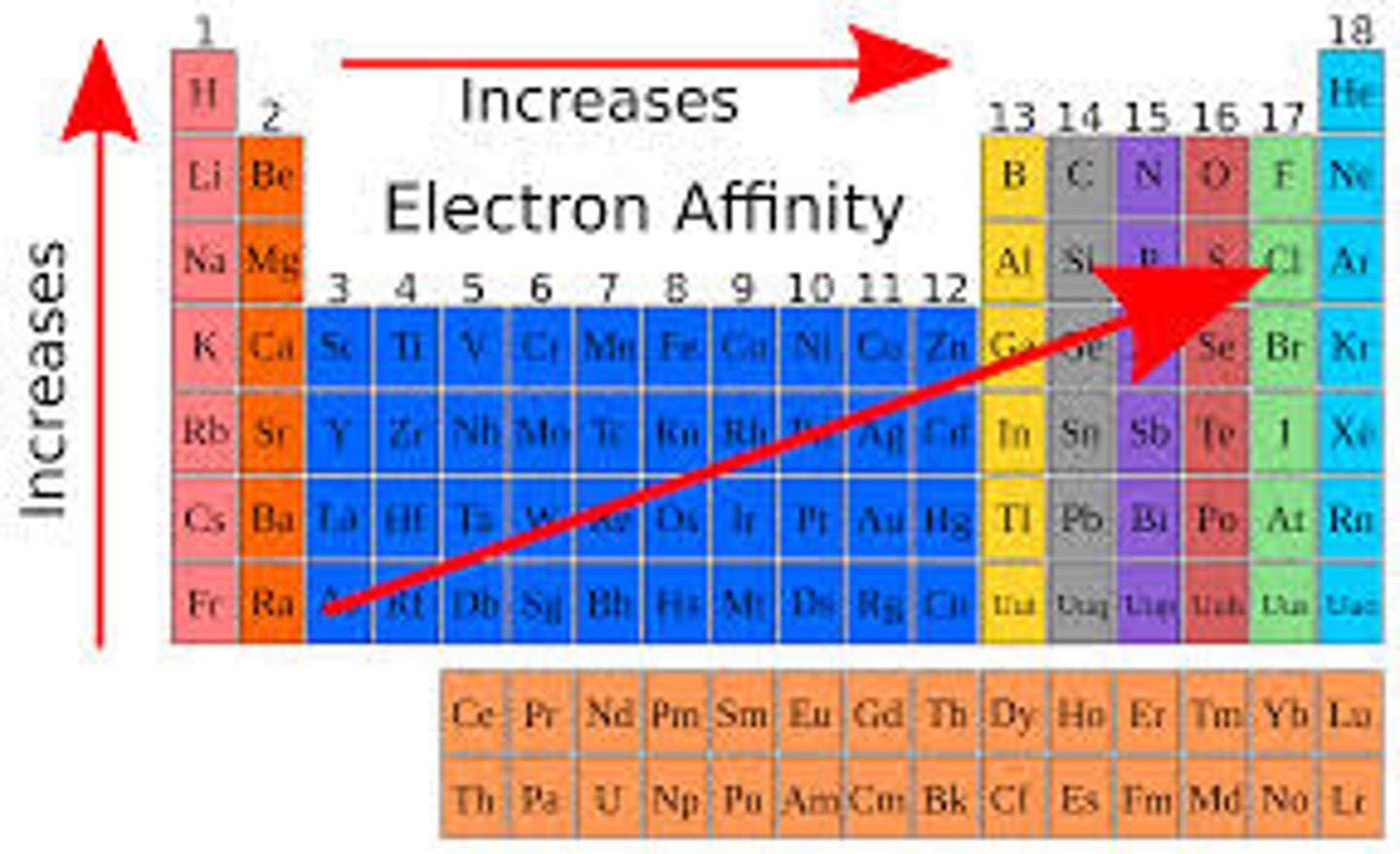
electron affinity trend
increases to the right and up
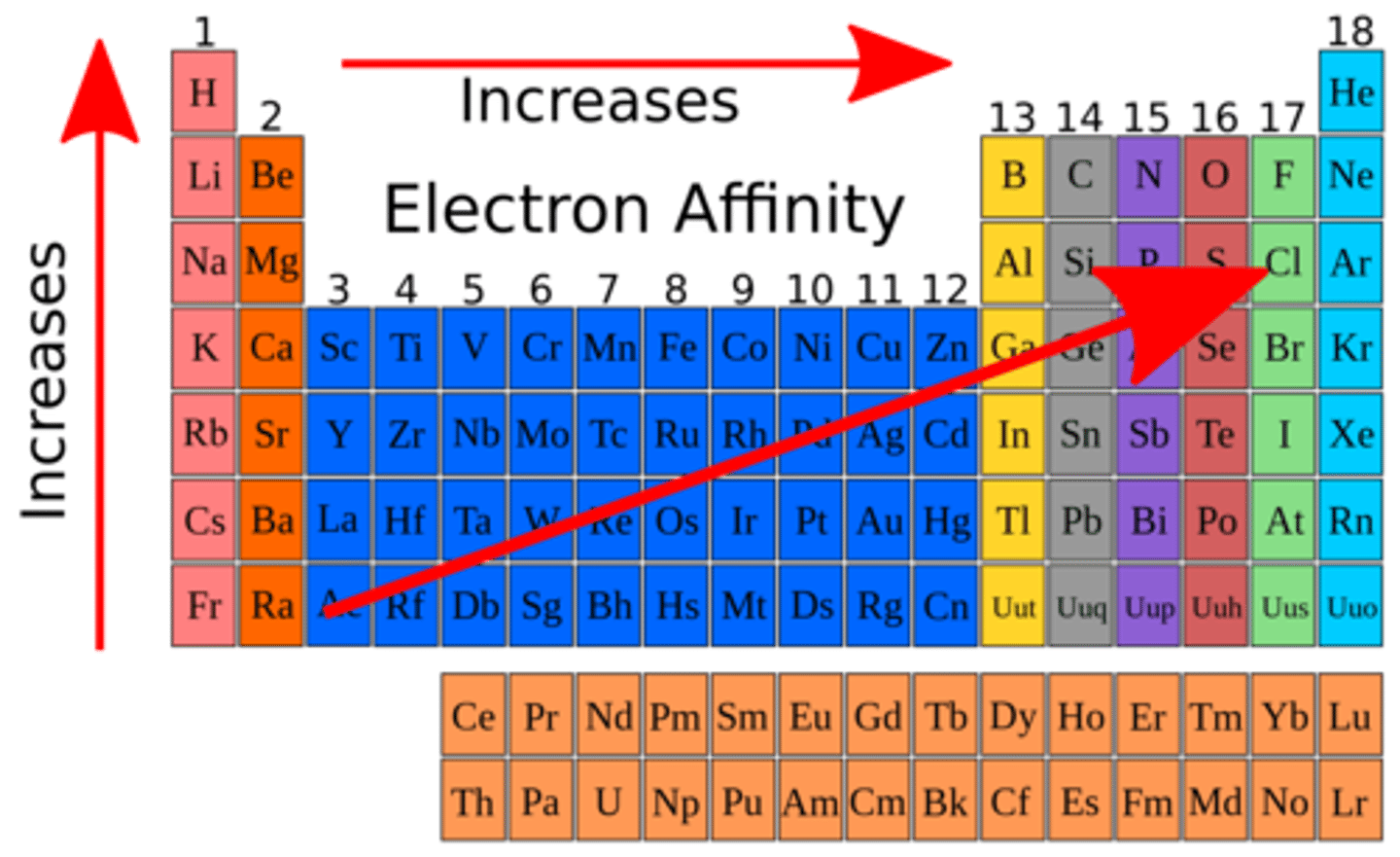
electronegativity
an atom's ability to attract electrons when bonded
electronegativity trend
increases up and to the right
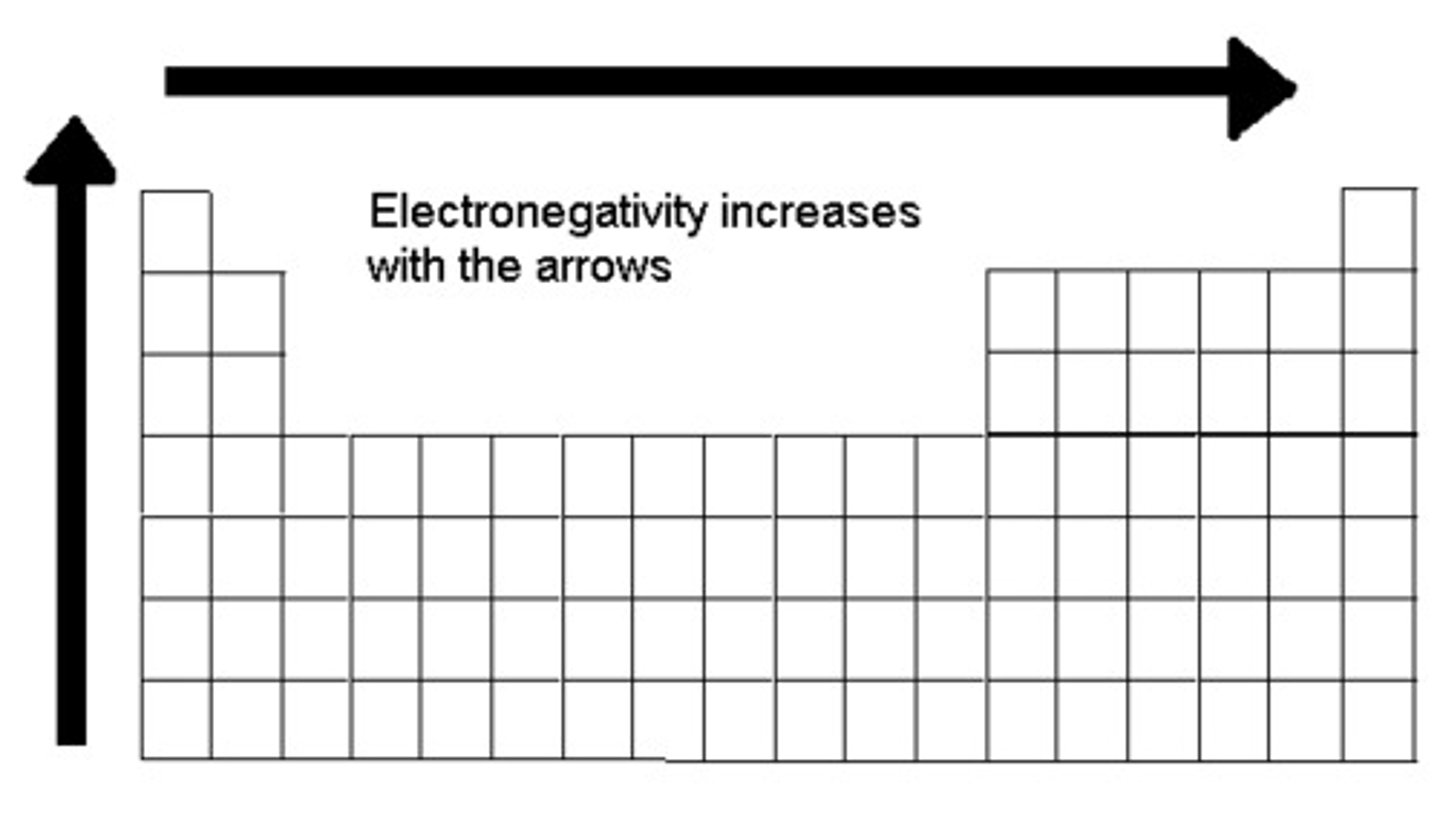
reason for electronegativity trend
when moving up, there is less electron shielding which promotes the effective nuclear charge, when moving left to right there are more protons which also increases the effective nuclear charge
properties of ionic compounds
crystalline structure, high melting and boiling points, very strong bond, brittle, and electrolyte can dissolve in water to make conductive solution
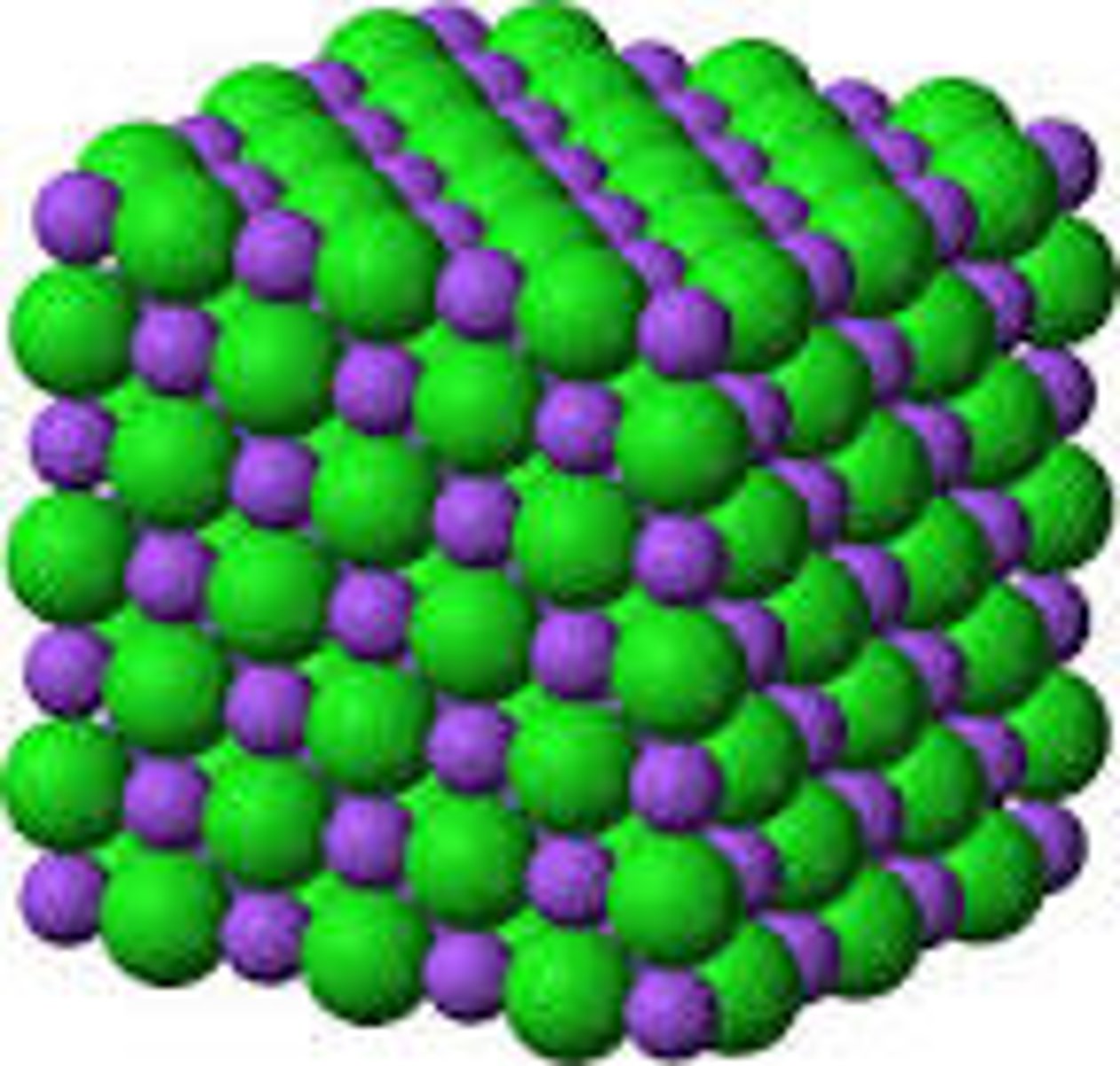
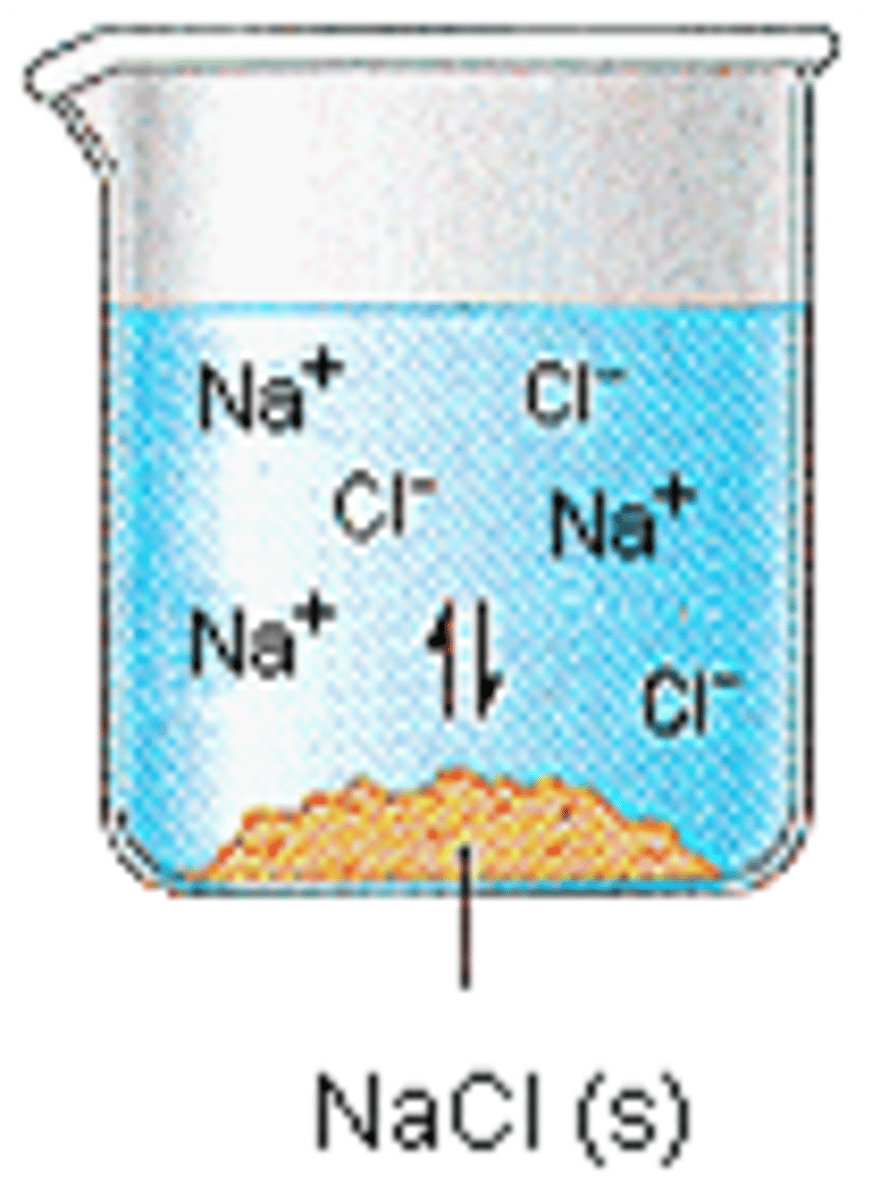
ionic electrolyte
individual ions will break up in solution, both positive and negative charges floating around
ionic bond
formed when one or more electrons are transferred from one atom to another to be stable
lewis diagram for ionic compounds
include brackets if ions, only express valence electrons on non-metals
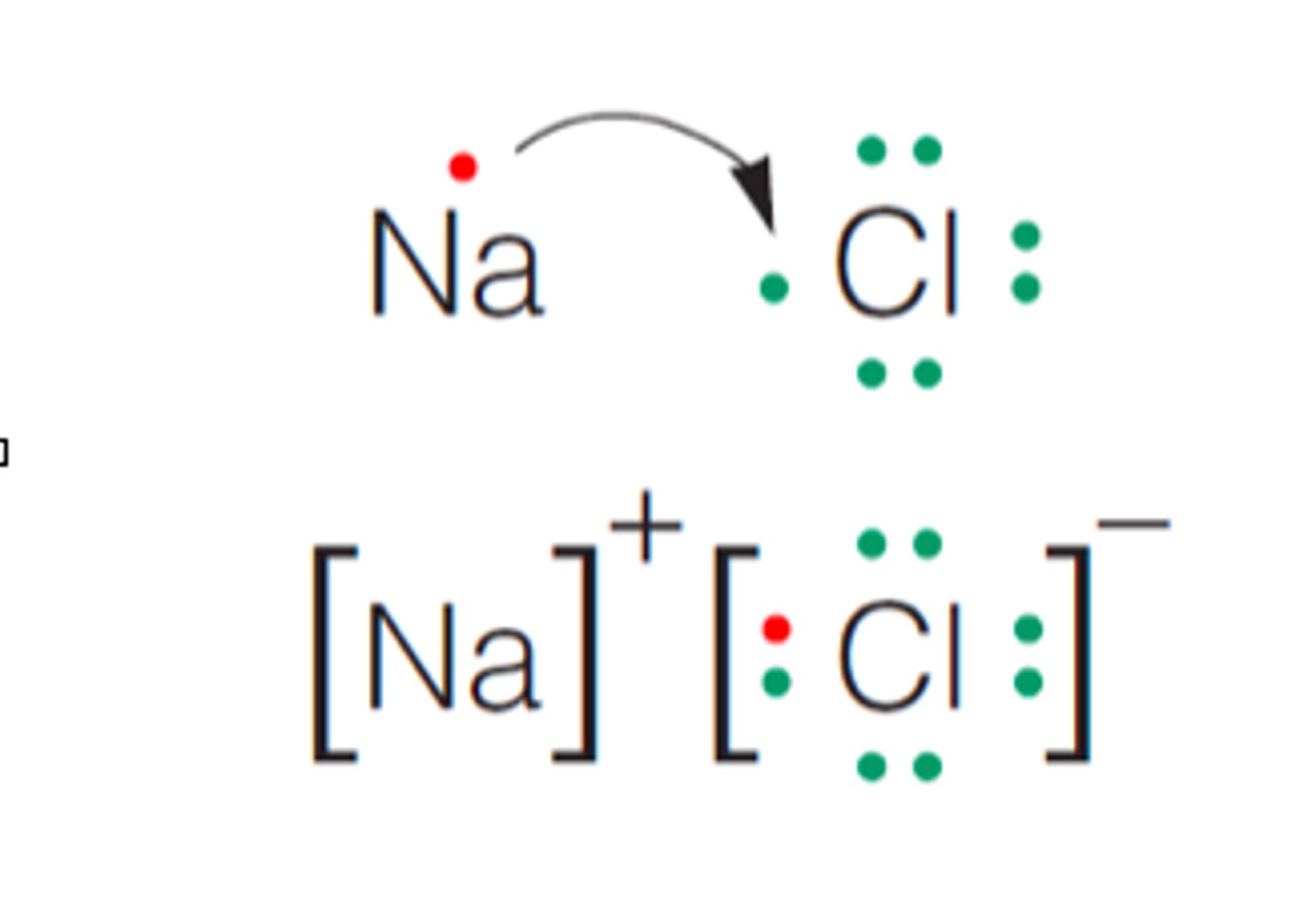
molecular compound
a covalent bond between non-metals
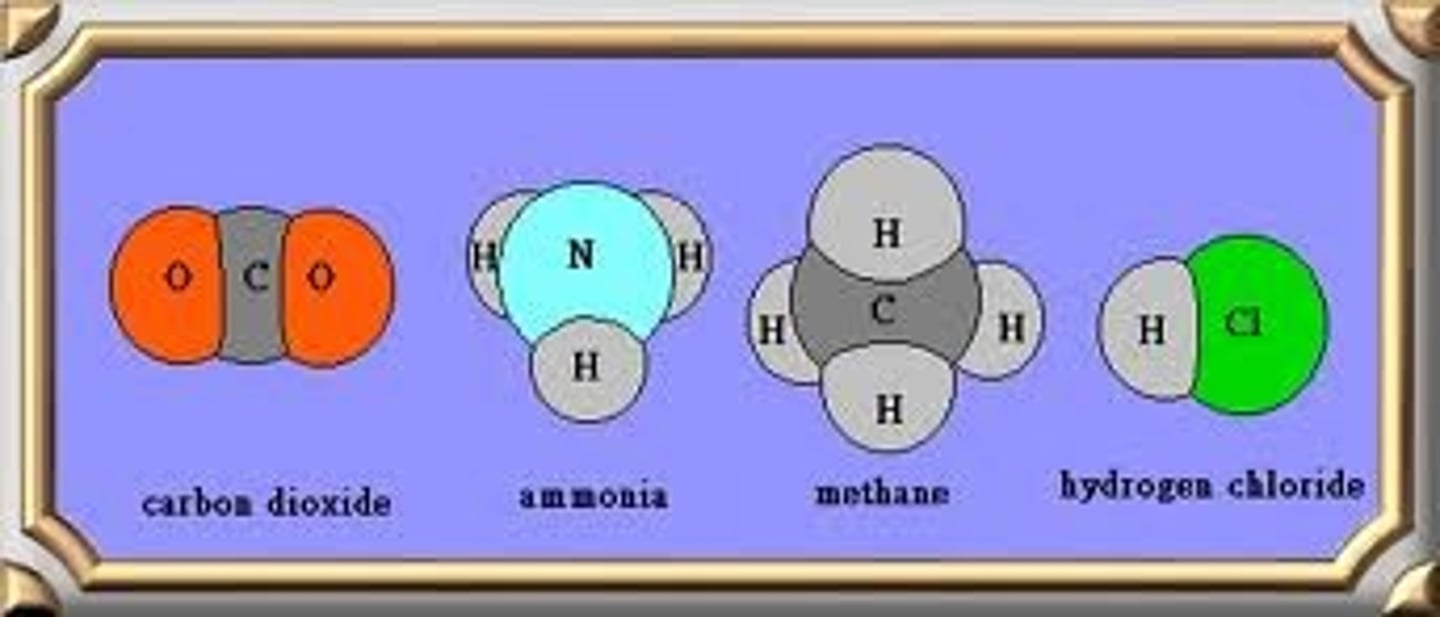
covalent bond
a bond in which the electrons are shared in order to become stable
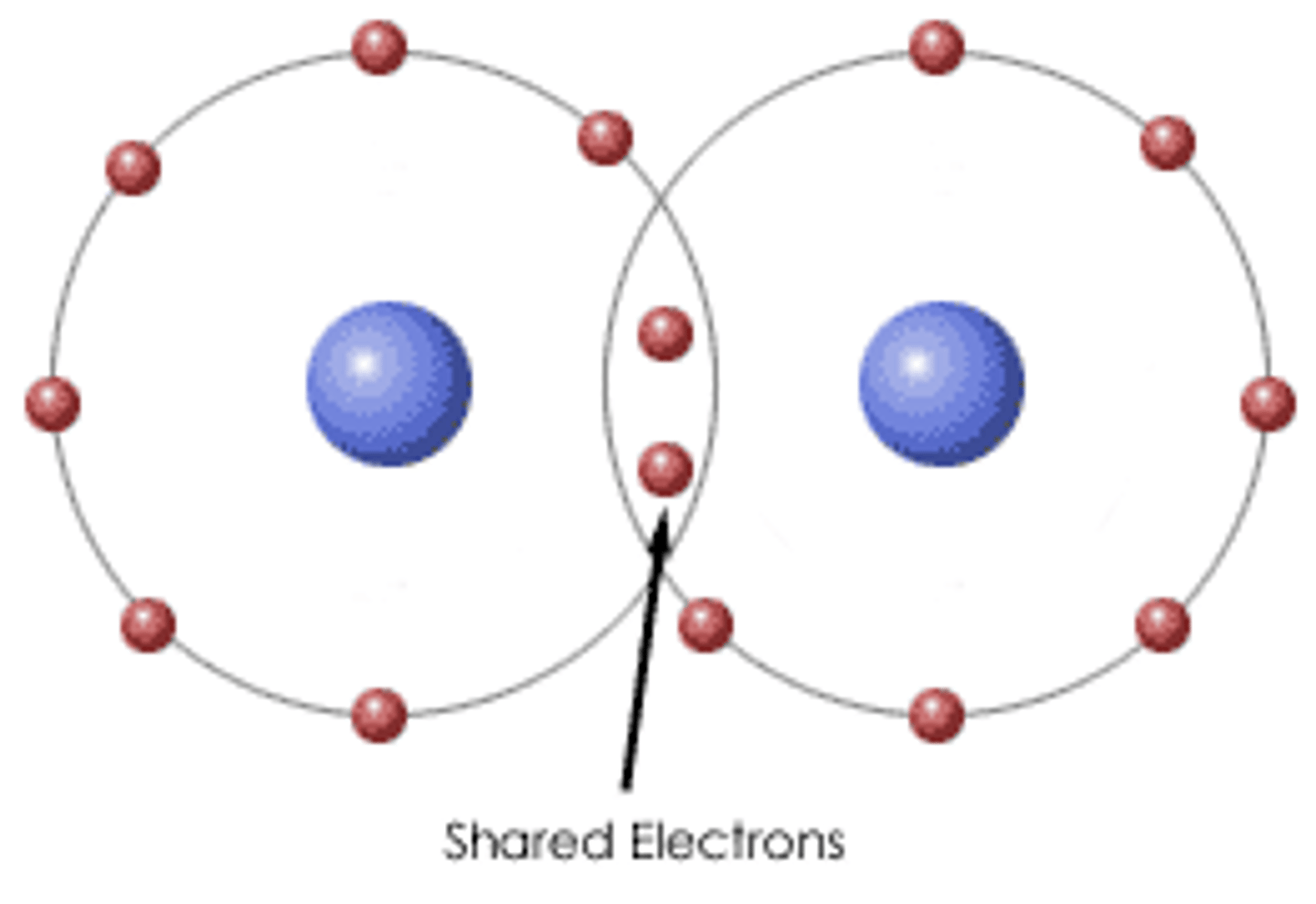
molecular compound properties
low melting and boiling points, low solubility as most will not dissolve in water, not conductive in when solutes, weaker bonds
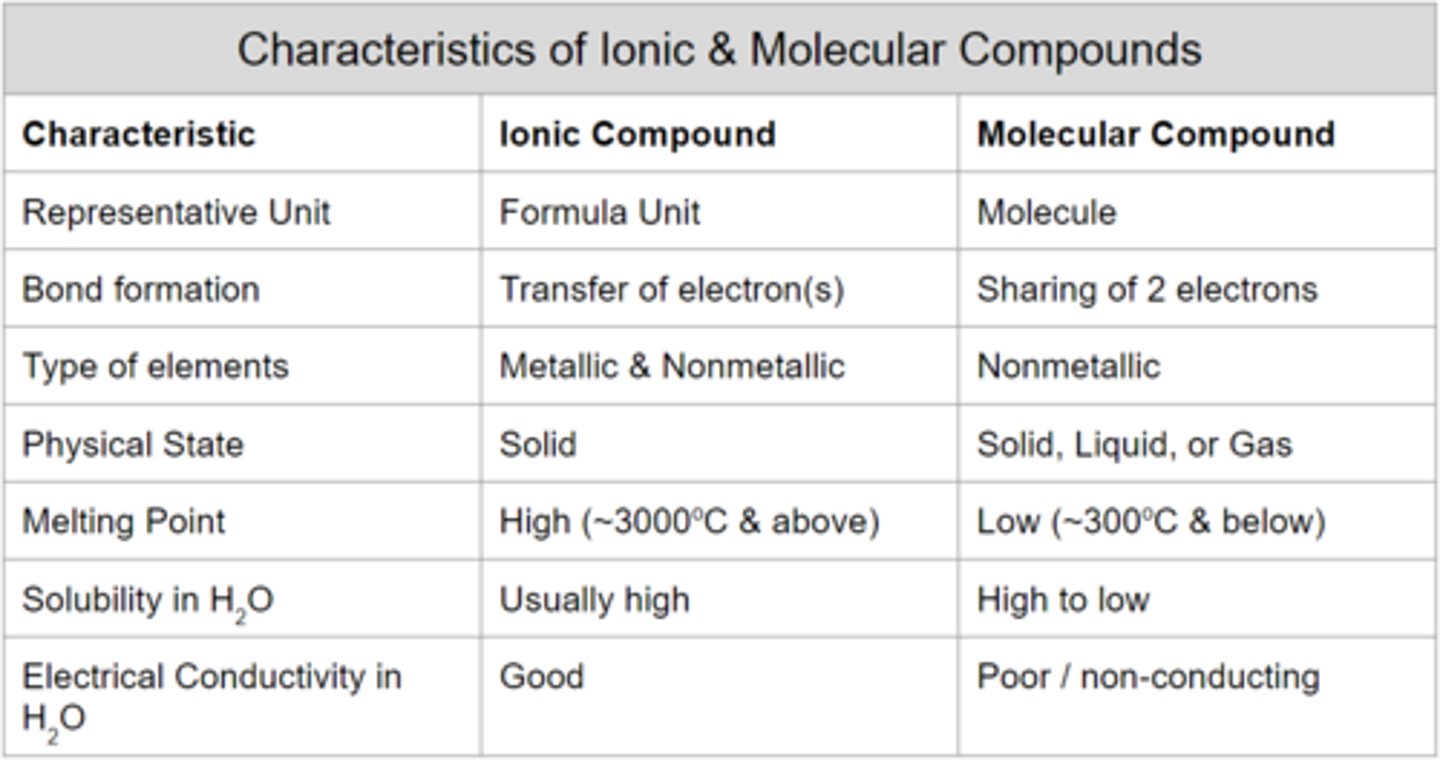
duet & octet rule
hydrogen is stable with two valence electrons, whilst both other elements are stable with eight
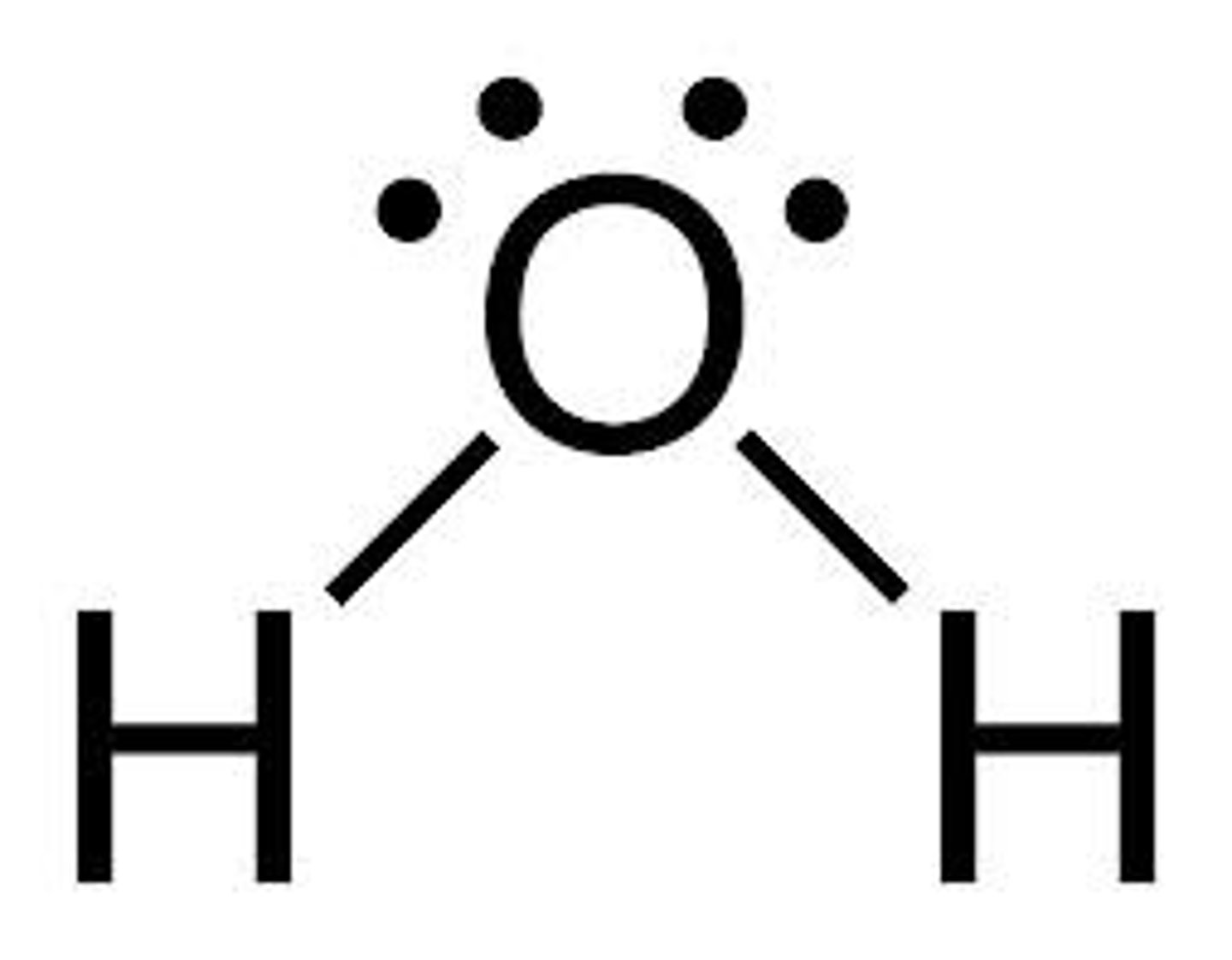
lewis structure for molecular compounds
central atom has highest bonding capacity, count up total electrons and show sharing of electrons
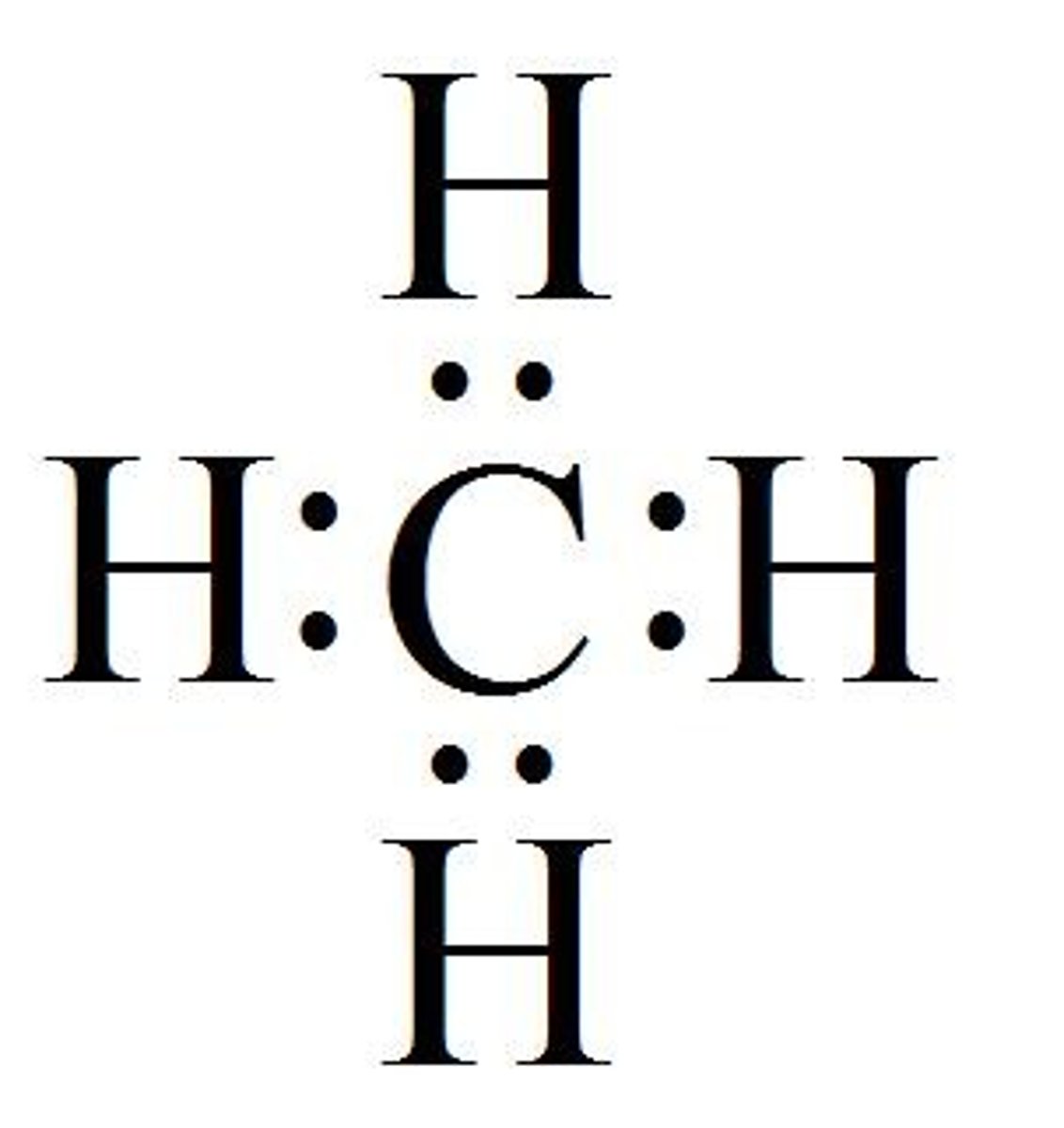
coordinate covalent bond
one atom contributes to both electrons being shares
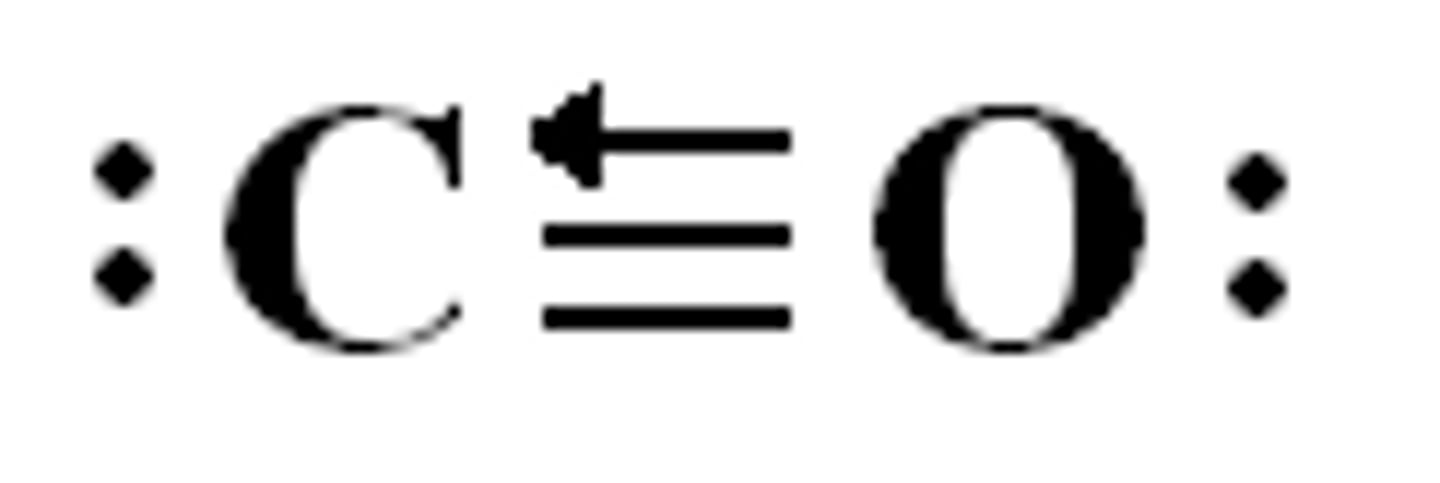
polarity
molecules having either an even or uneven distribution/sharing of electrons when in structure
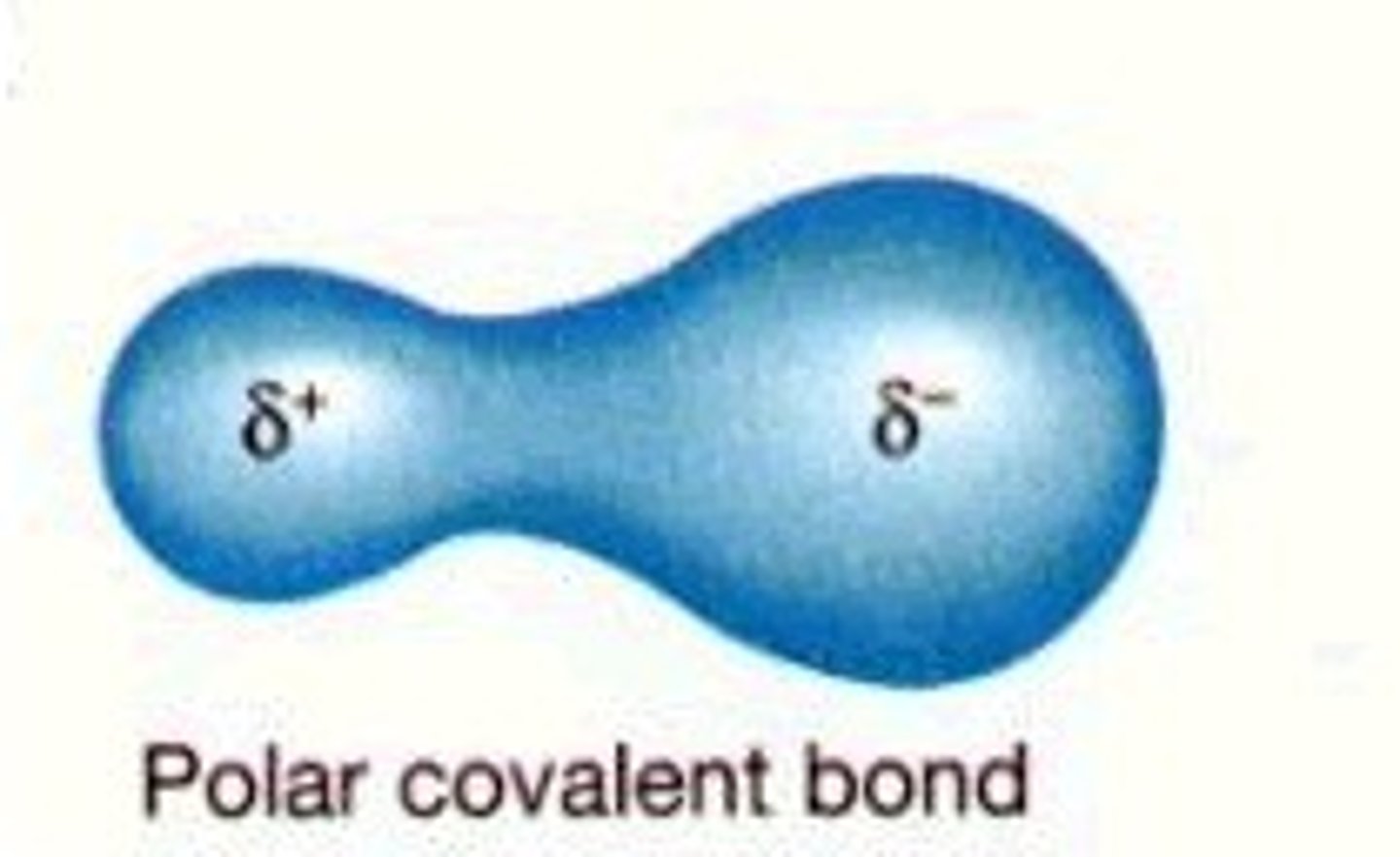
polar covalent bond
a covalent bond in which the electrons are not being shared equally, thus being pulled closer to the atom with a higher electronegativity
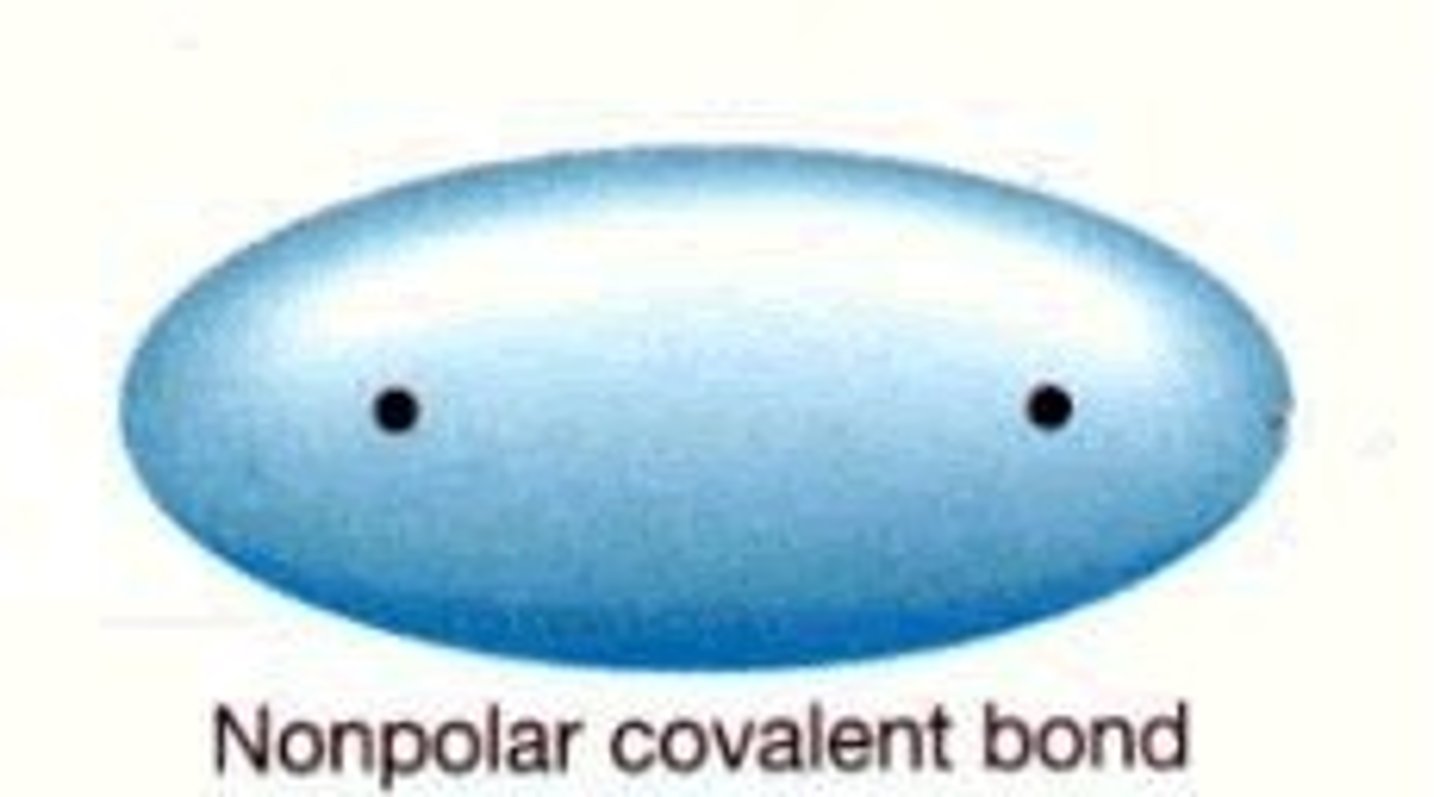
non-polar covalent bond
a covalent bond in which the electrons are being shared equally
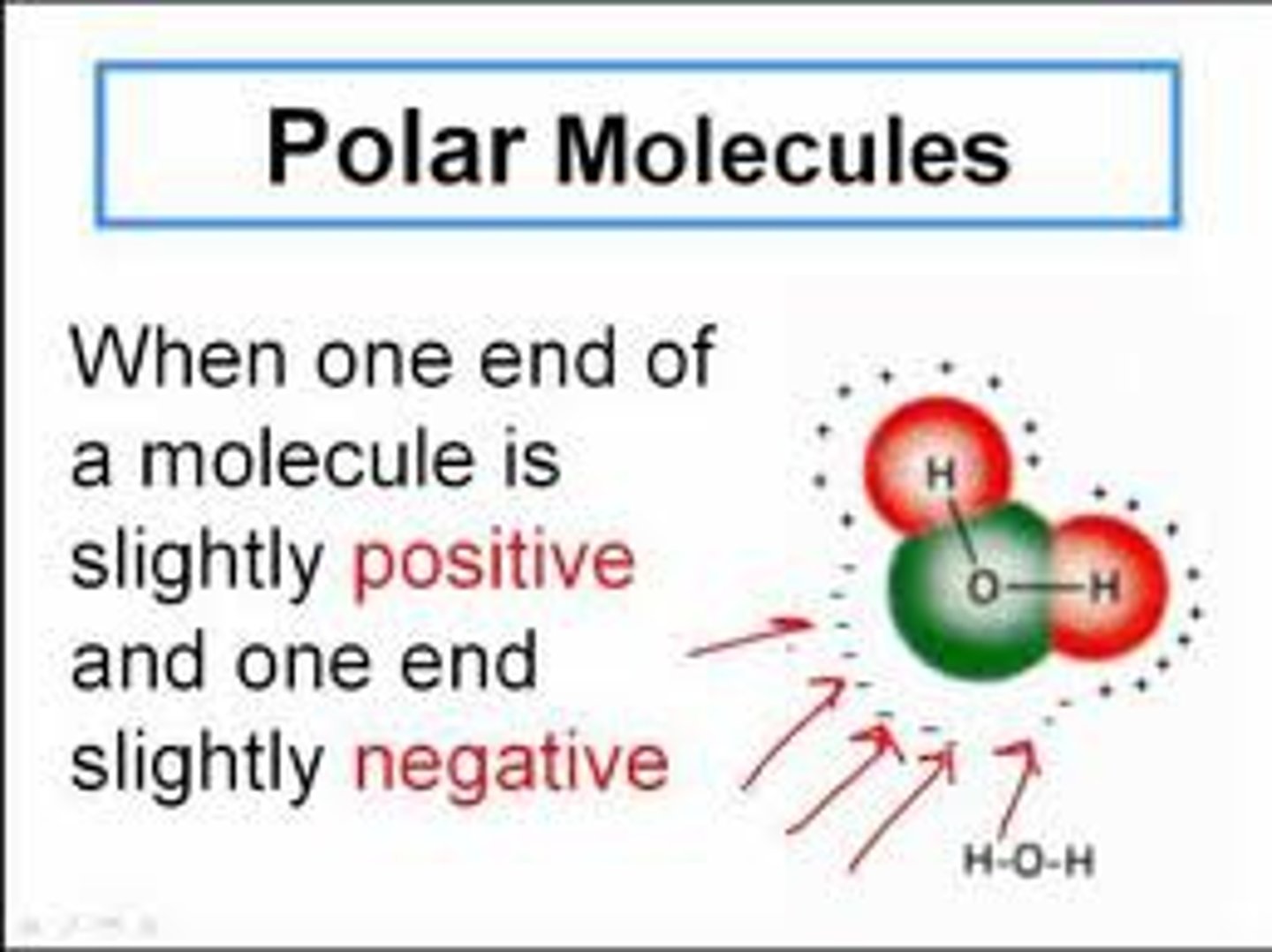
polar molecule
a molecule that not only has a polar covalent bond, but that is also asymmetrical
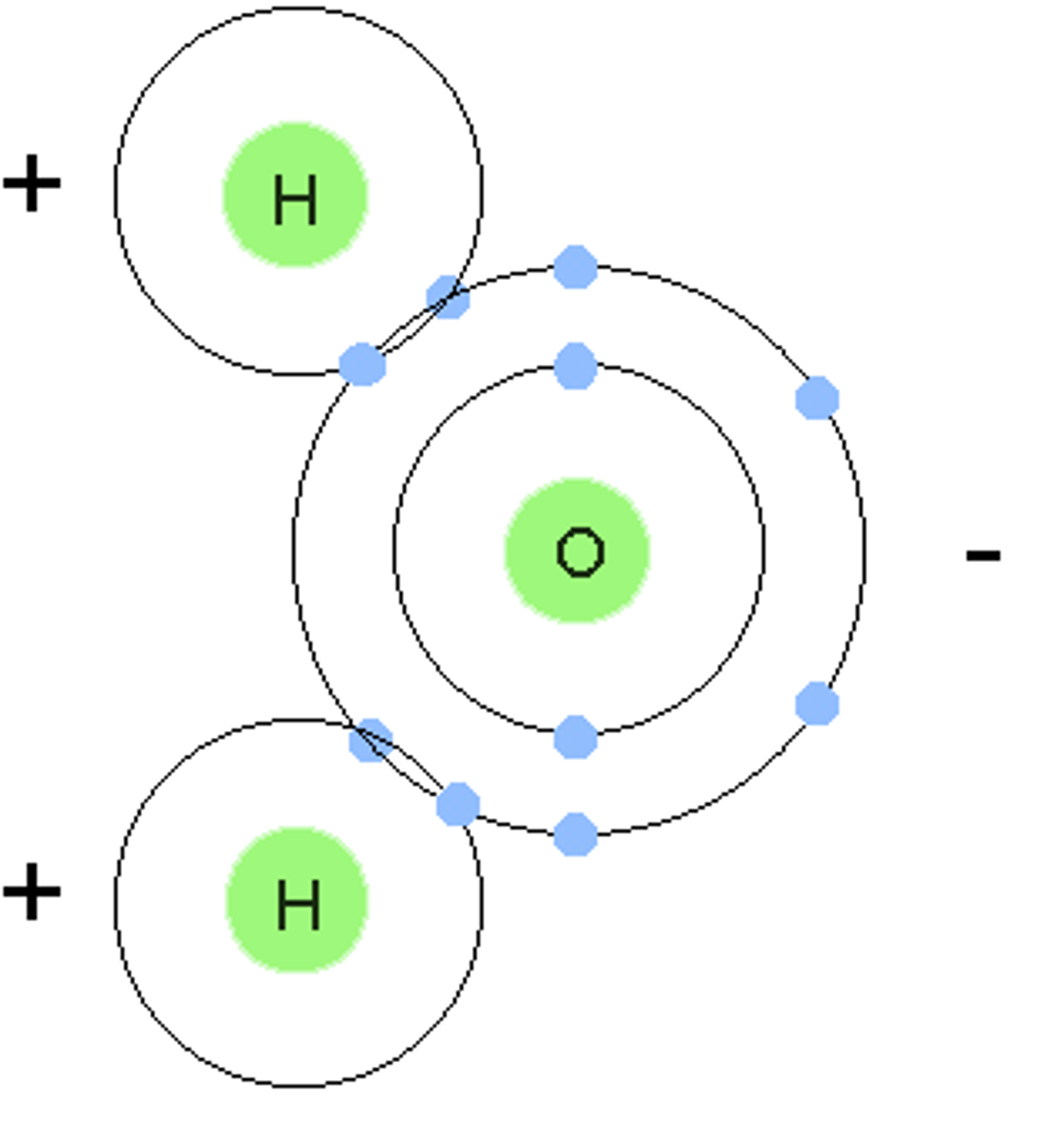
non-polar molecule
a molecule that not only has a polar covalent bond, but that is symmetrical at all bonding sites
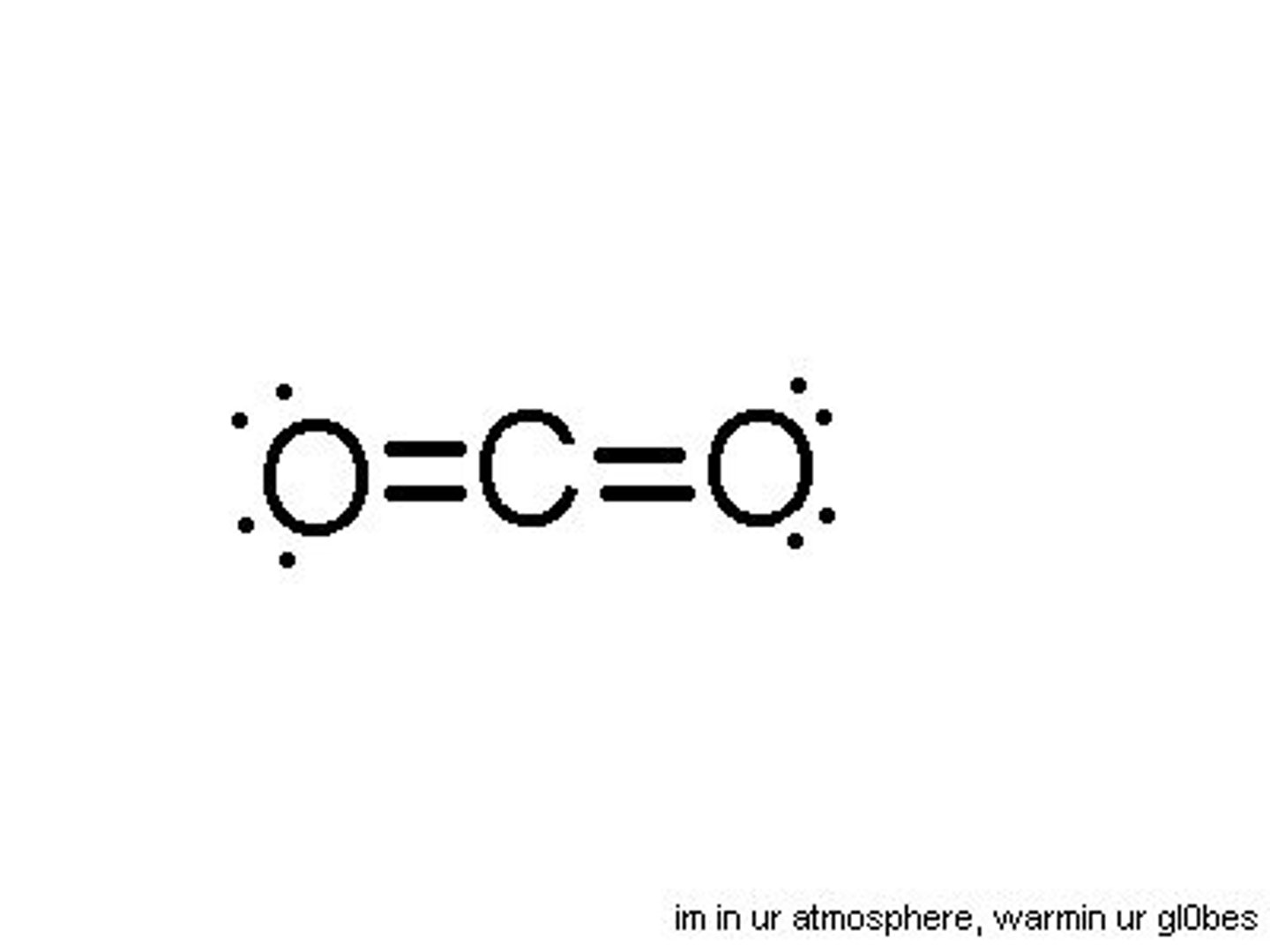
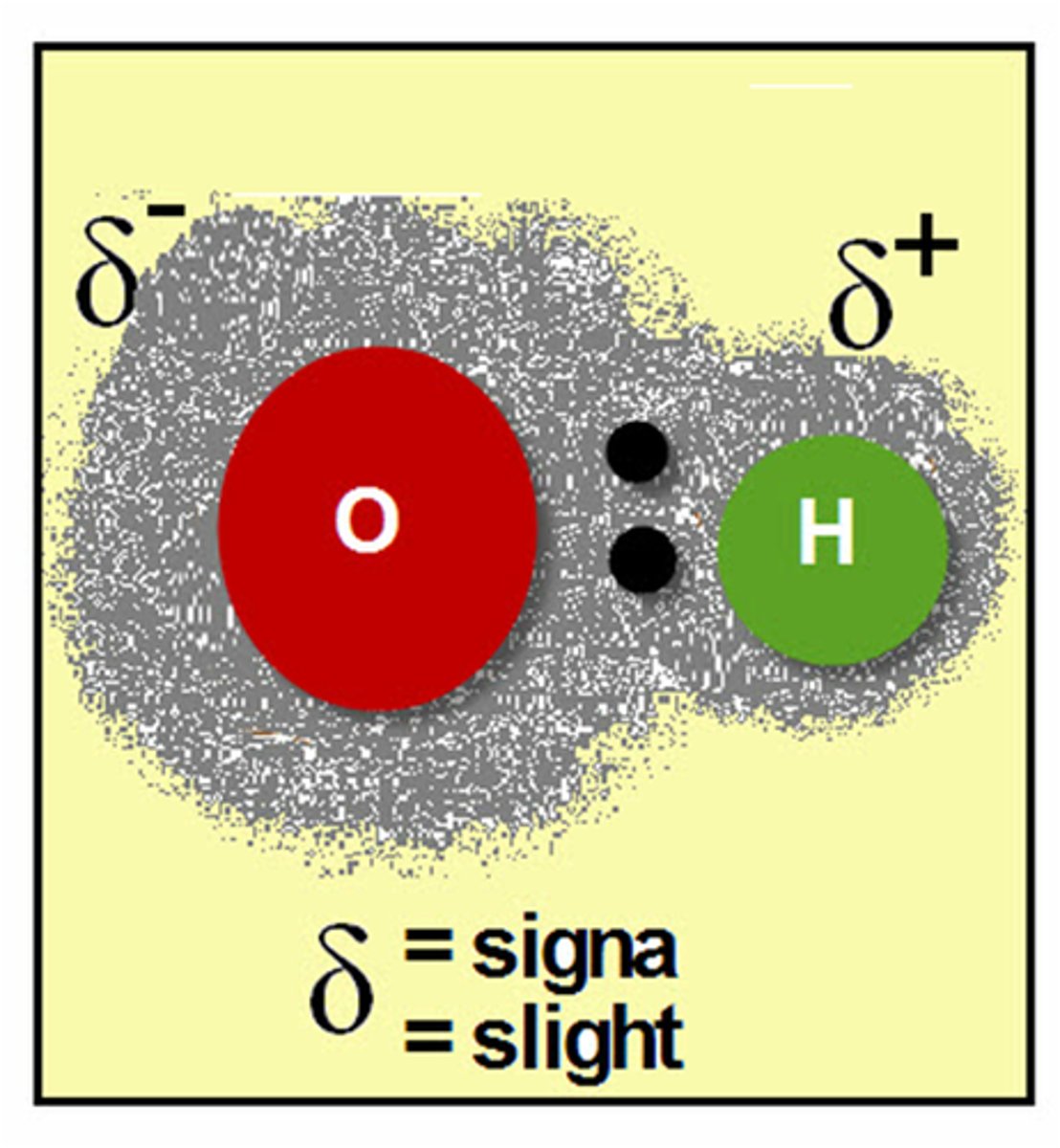
relative positive charge
the atom in a polar covalent bond that is not pulling the electrons closer, represented as delta +
relative negative charge
the atom in a polar covalent bond that is pulling the electrons closer, represented as delta -
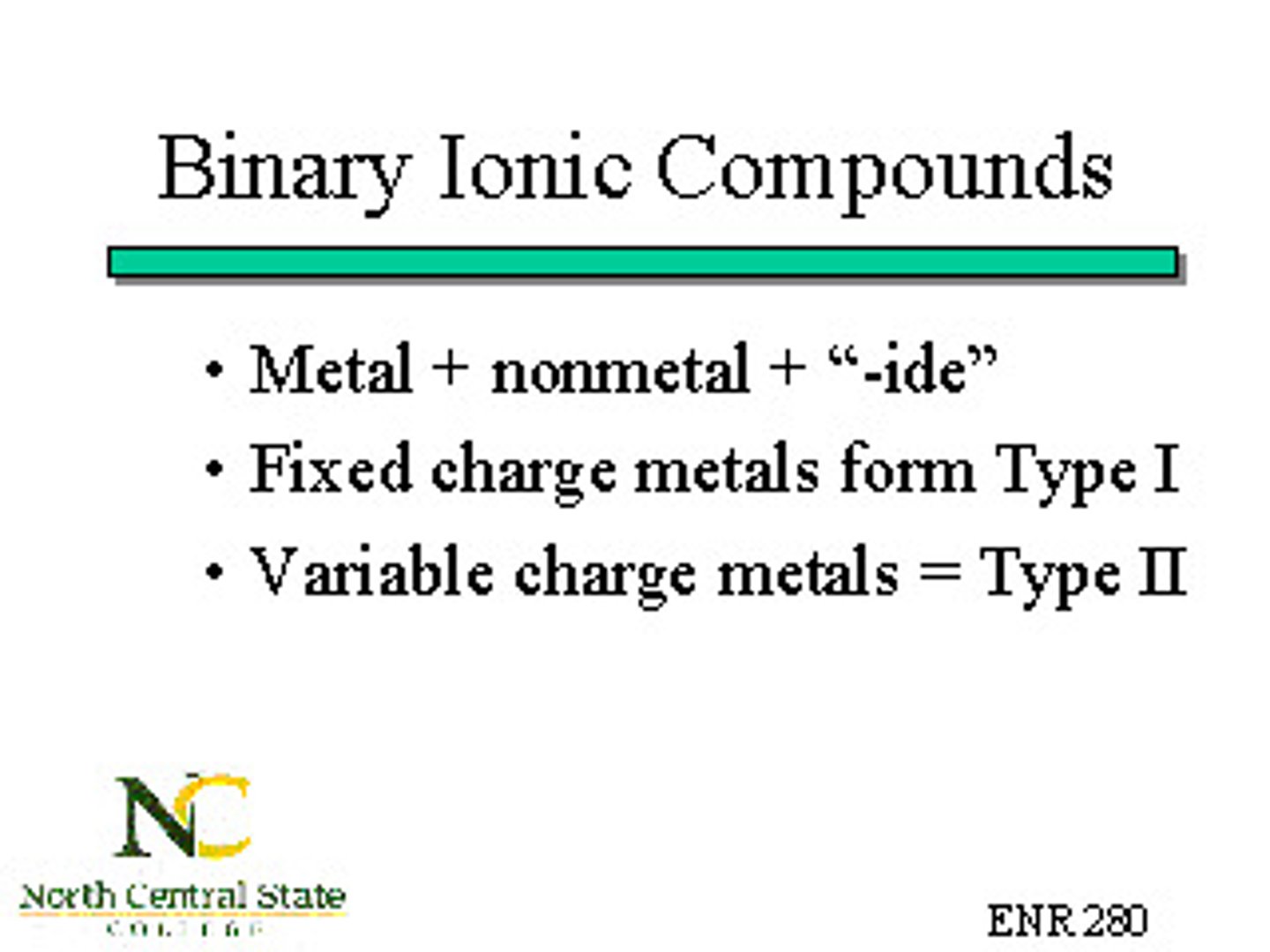
binary ionic compound
bond between a metal and non-metal, first element stays the same and second element ends in -ide
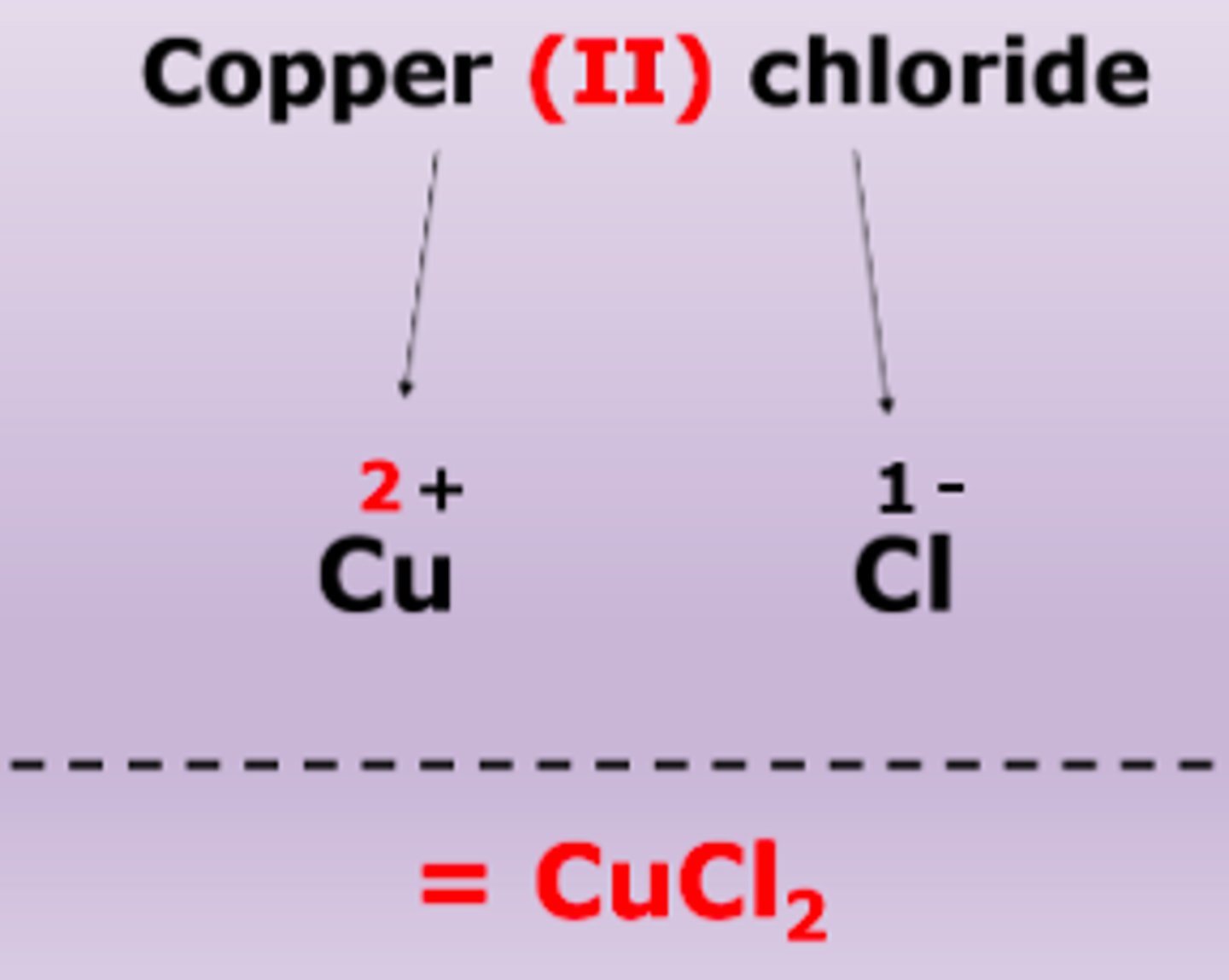
multivalent ionic compound
bond between metal and non-metal, first element stays the same but includes roman numeral to indicate charge, second element ends in -ide
oxyanion
polyatomic ion composed of oxygen

oxyacid
polyatomic ion with hydrogen at start (remember aq in formula writing)
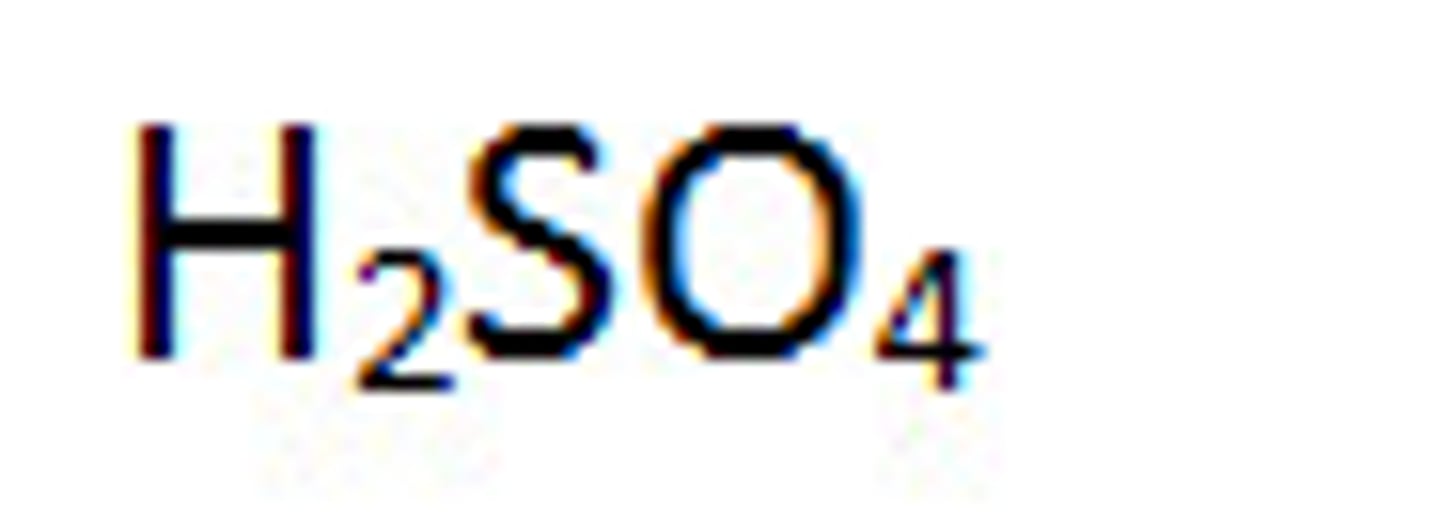
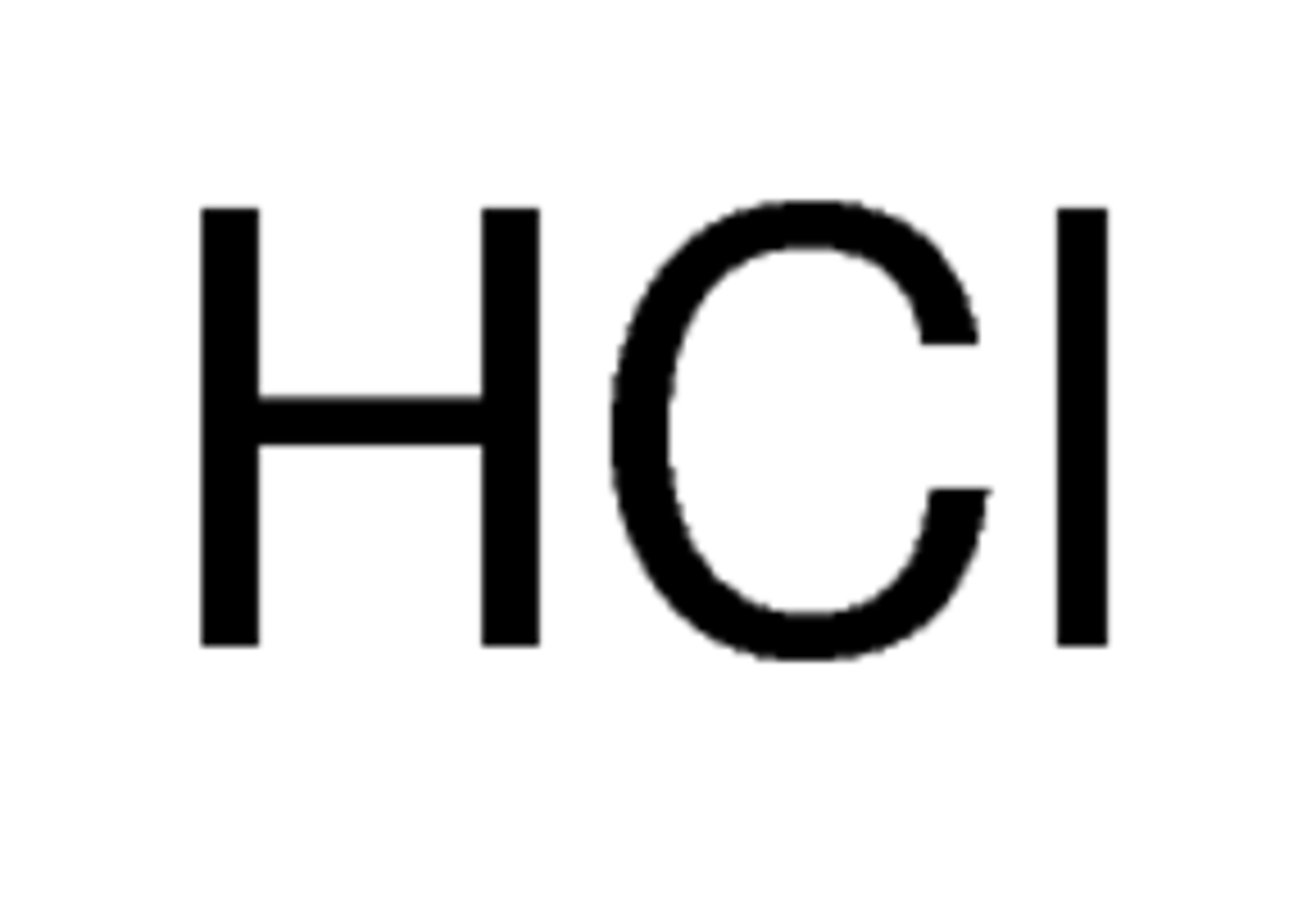
acid
hydrogen and non-metal (remember aq in formula writing)
base
metal and hydroxide (remember aq in formula writing)
hydrate
ionic compound with water molecules stuck to its structure, - molecular prefix + hydrate

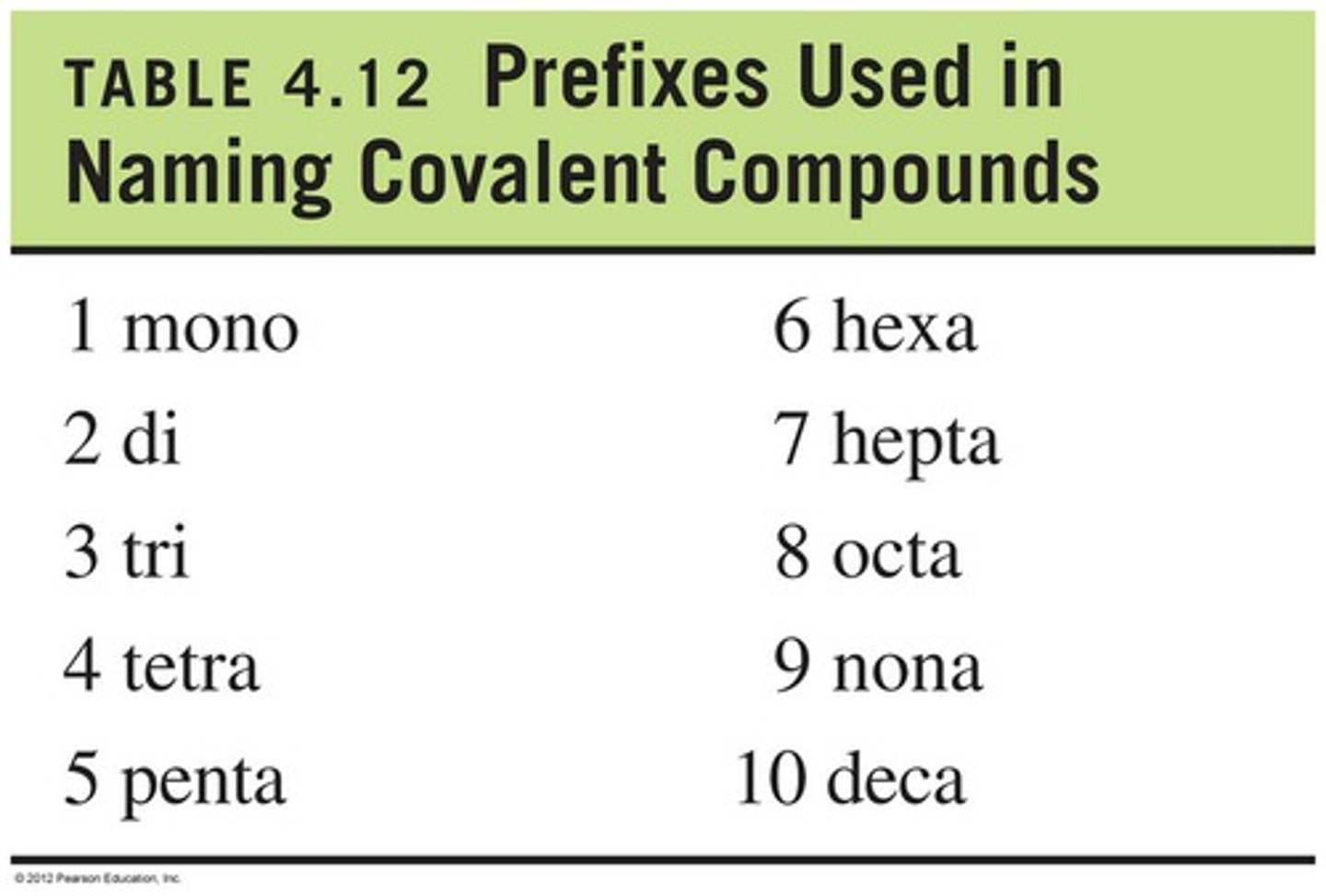
molecular prefixes
1-mono
2-di
3-tri
4-tetra
5-penta
6-hexa
7-hepta
8-octa
9-nona
10-deca
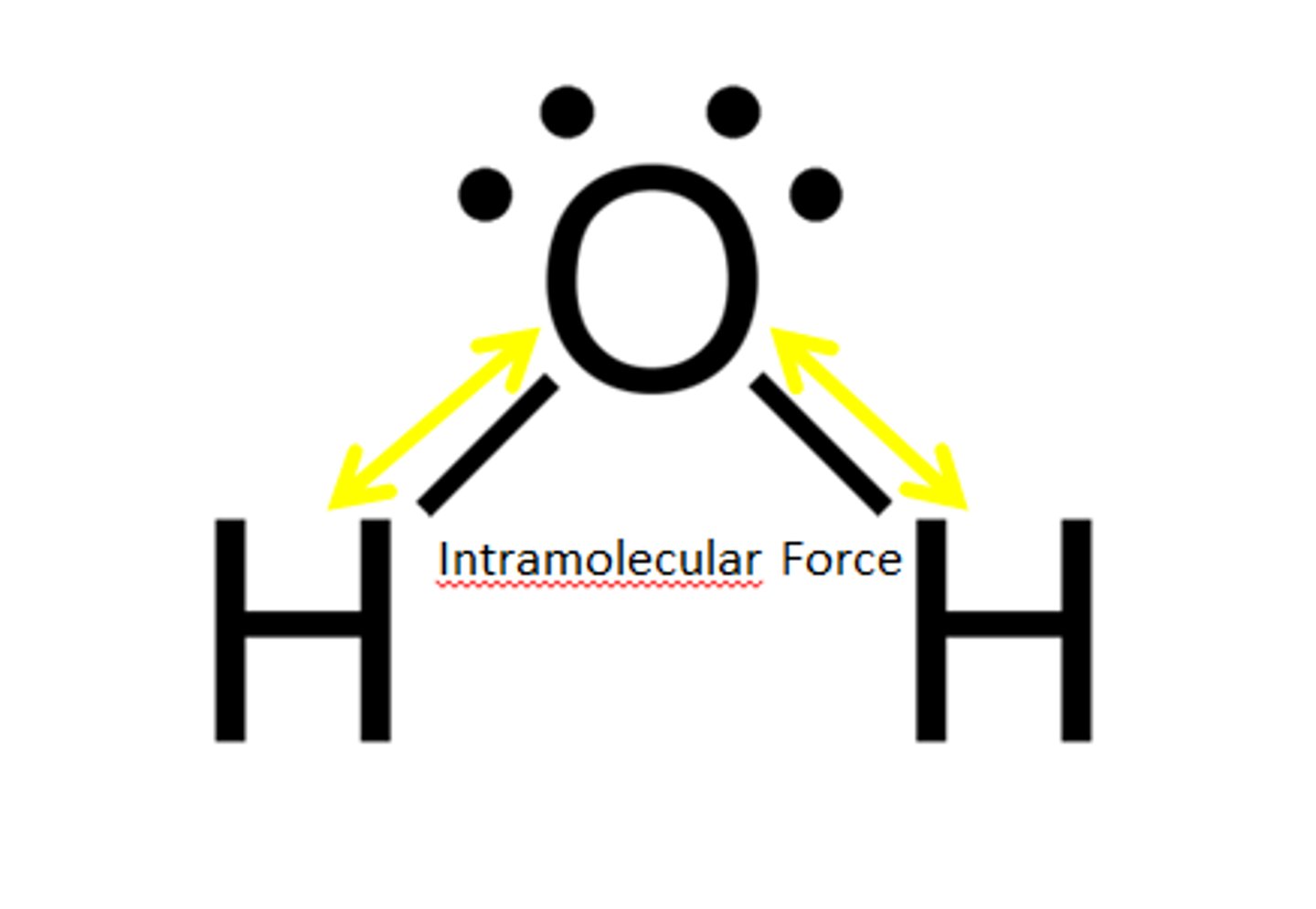
intramolecular forces
forces within molecules
intermolecular forces
forces between molecules
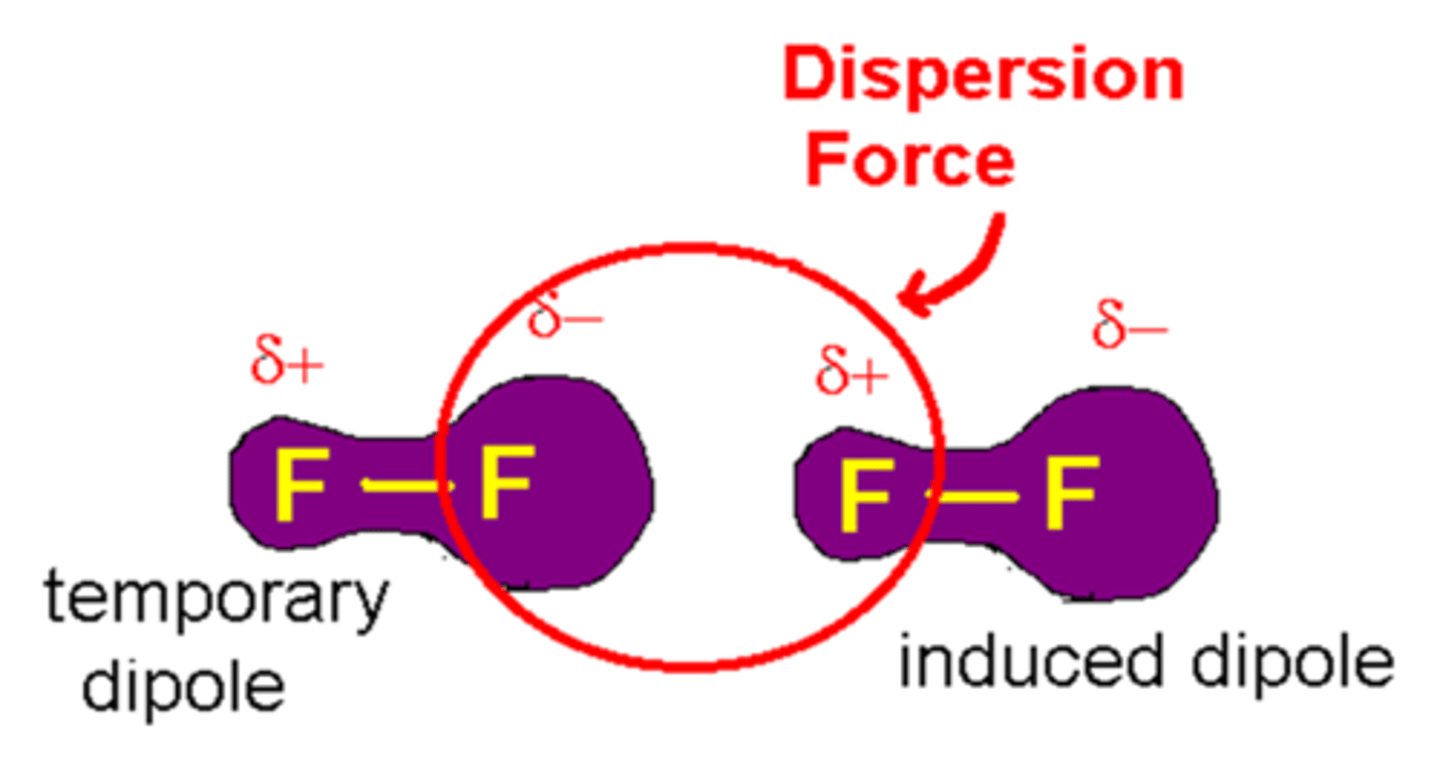
london dispersion force
in all molecules, it is the slight attraction of an atom's positive nuclei to neighbouring electrons
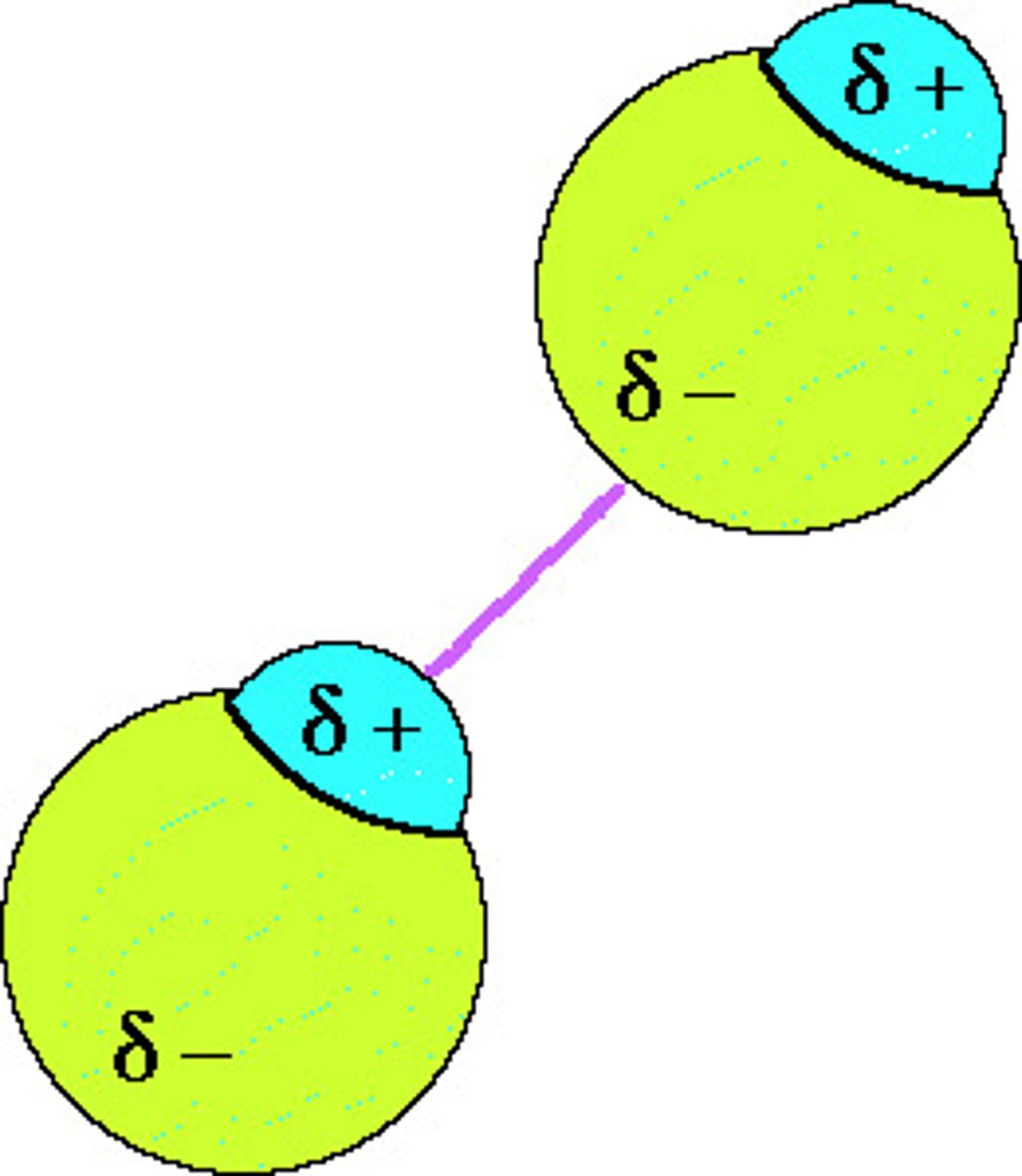
dipole-dipole
in only polar molecules, attracted force between positive and negative end of molecule caused by relative charges, it is the stronger than LDF, but weaker than hydrogen bonding
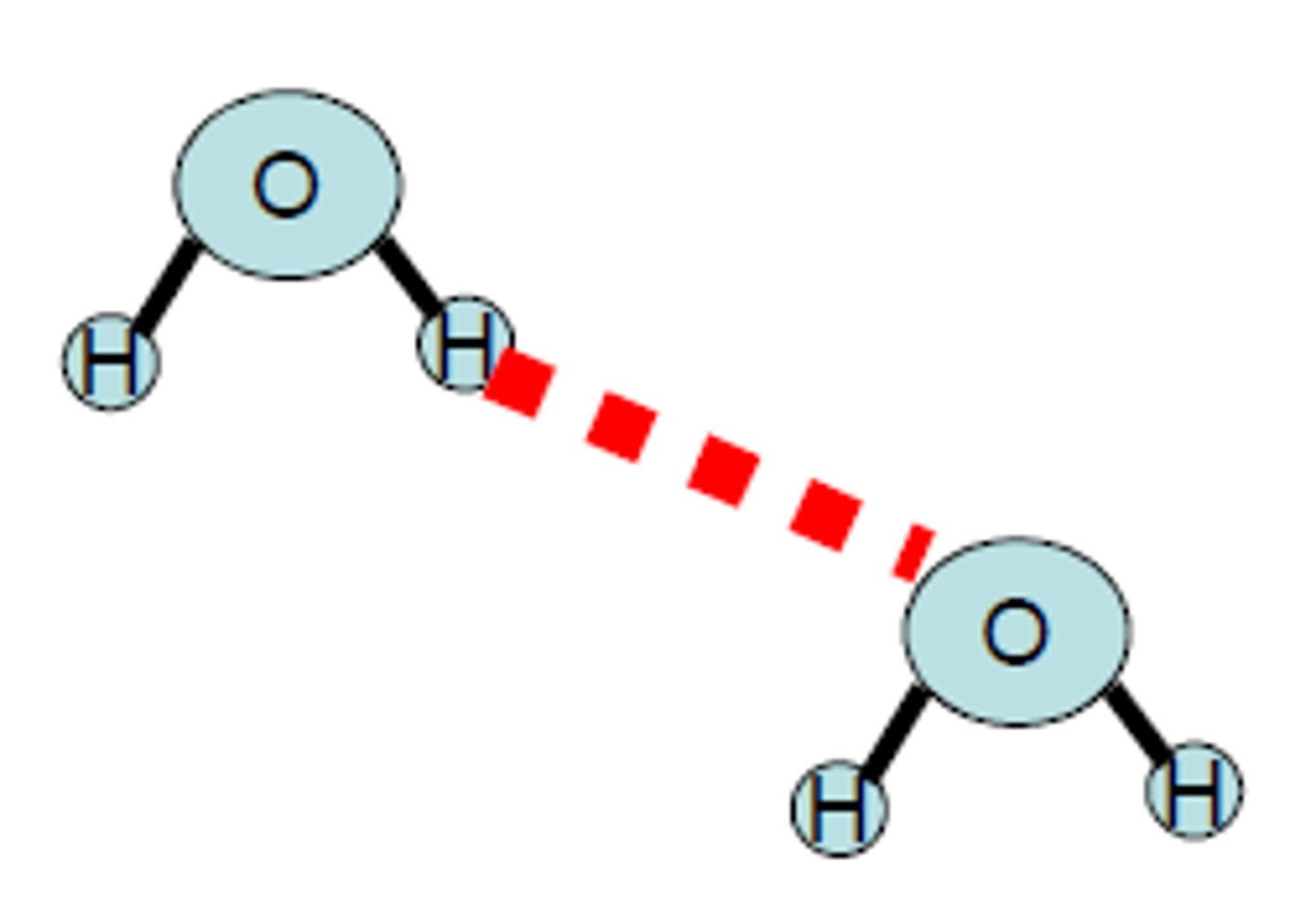
hydrogen bonding
stronger dipole-dipole force with relatively positive charged hydrogen attached to either N, O, or F
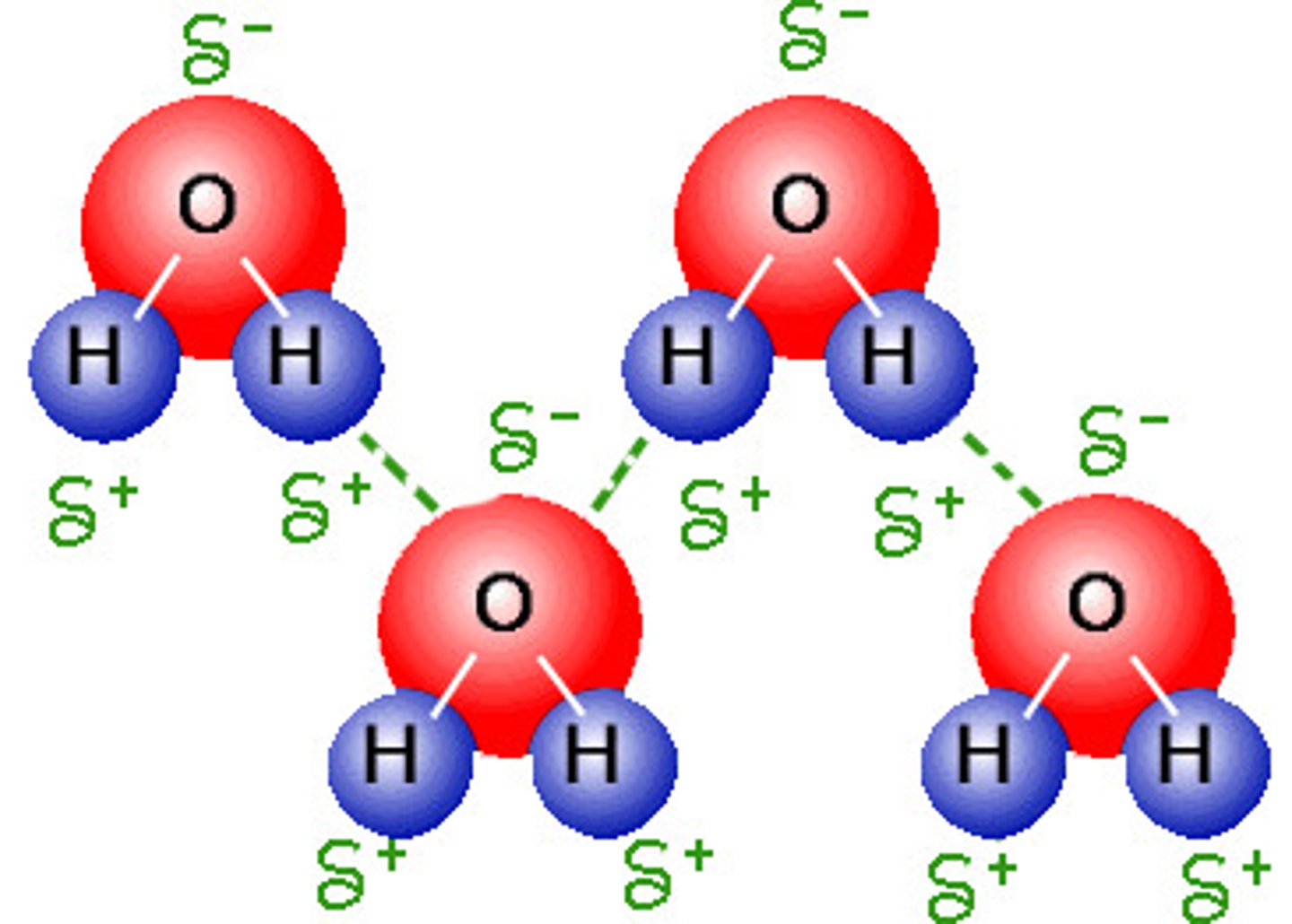
relative boiling points
as intermolecular forces increase, boiling point and surface tension increase
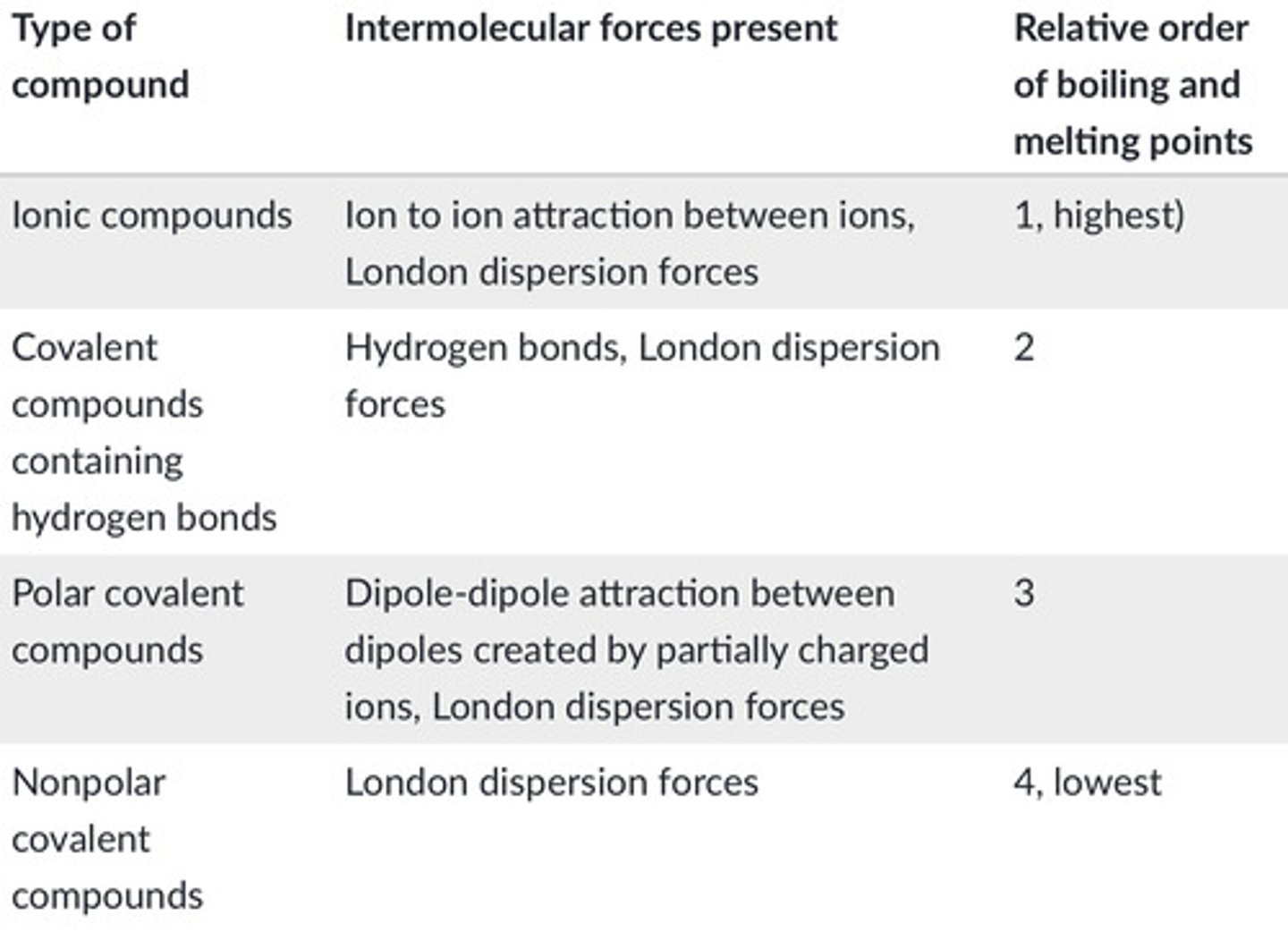
order of intermolecular forces by strength
weakest to strongest: LDF, dipole-dipole, hydrogen bonding, ionic bonding
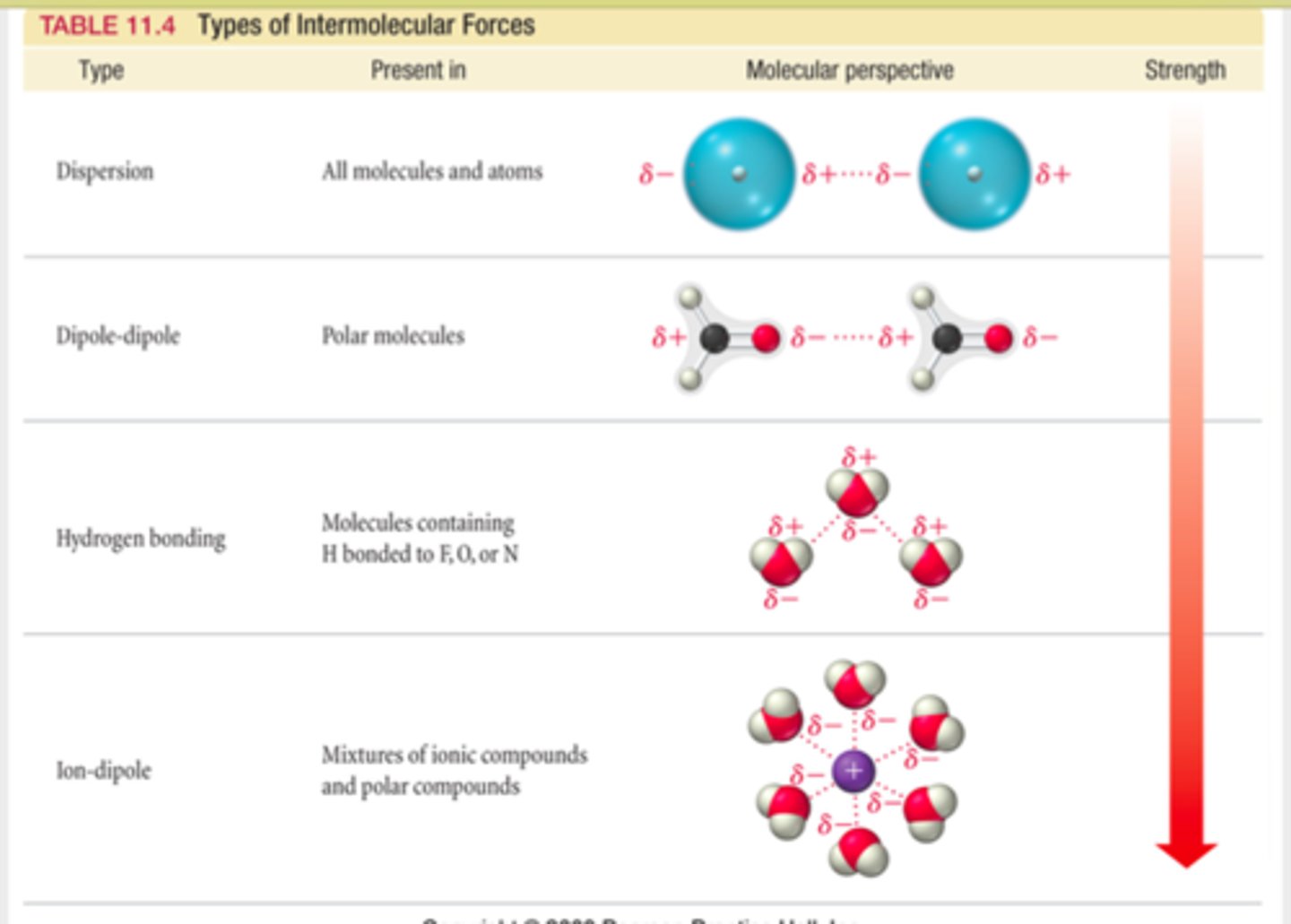
LDF differentiation
the strongest LDF will be the largest molecule, then the one with more electrons, anything following that
standard atomic notation
mass number in top left, atomic number on bottom left