2.2.1 Electron Structure
0.0(0)
Card Sorting
1/16
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
1
New cards
What does the Bohr model of the atom describe?
Electrons in fixed spherical shells (2,8,8) around the nucleus.
2
New cards
What is the limitation of the Bohr model?
It does not explain sub-shells or the shapes of orbitals.
3
New cards
What are the four sub-levels and their electron capacities?
s (2), p (6), d (10), f (14).
4
New cards
What is an orbital?
A region of space where there is a high probability of finding an electron.
5
New cards
How many electrons can each orbital hold?
2 electrons with opposite spins.
6
New cards
What is the shape of an s-orbital?
Spherical.
7
New cards
What is the shape of a p-orbital?
Dumbbell-shaped.
8
New cards
What is Hund’s rule?
Orbitals in the same sub-level are filled singly before pairing.
9
New cards
What is the order of filling orbitals up to 5p?
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p.
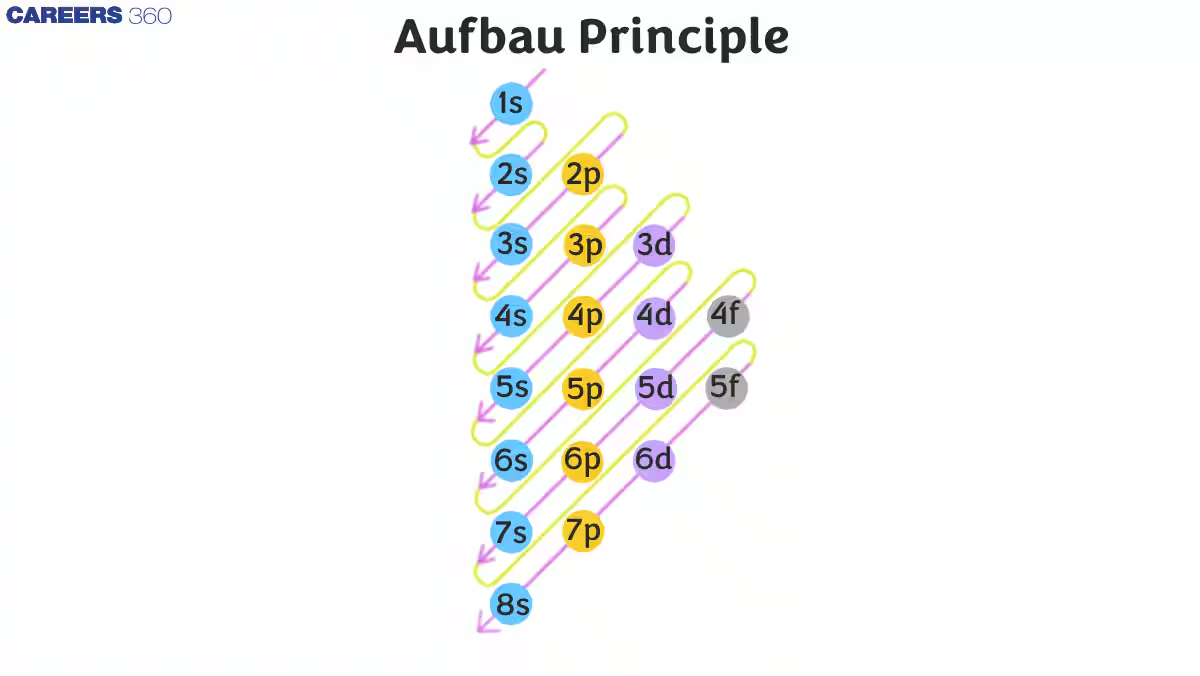
10
New cards
Why does 4s fill before 3d?
Because the 4s sub-level is lower in energy than 3d.
11
New cards
Which electrons are lost first when transition metals form ions?
The 4s electrons.
12
New cards
How many orbitals are in the 2p sub-level?
3 orbitals (each holding 2 electrons).
13
New cards
How many orbitals are in the 3d sub-level?
5 orbitals (each holding 2 electrons).
14
New cards
What determines the block (s, p, d, f) of an element in the periodic table?
The type of orbital that the highest energy electron occupies.
15
New cards
Why are noble gases chemically unreactive?
They have a full outer electron shell (stable configuration).
16
New cards
Exam Q: Which sub-level is filled after the 5s orbital?
4d.
17
New cards
Exam Q: Why do electrons in the same orbital have opposite spins?
To reduce repulsion between them (Pauli exclusion principle).