Nomenclature of Saturated Hydrocarbons
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
IUPAC
International Union of Pure and Applied Chemistry
Alkanes and Cycloalkanes
belong to the family of saturated hydrocarbons
Alkanes and Cycloalkanes
molecules containing elements carbon and hydrogen connected by single bonds only
IUPAC Nomenclature
based on naming a molecule’s longest chain of carbon atoms connected by single bonds, whether in continuous chain or in a ring
Prefix
includes the kind and position of the substituents coming off the main chain
Parent
is the main chain
Parent Chain
the longest continuous chain of carbon atoms present in the molecule
Substituents
Groups that are not part of the longest chain are referred to as the branches or
Suffix
is the family
meth
methane = 1
eth
ethane = 2
prop
propane = 3
but
butane = 4
pent
pentane = 5
hex
hexane = 6
hept
heptane = 7
oct
octane = 8
non
nonane = 9
dec
decane = 10
undec
undecane = 11
dodec
dodecane = 12
tridec
tridecane = 13
tertradec
tertradecane = 14
pentadec
pentadecane = 15
hexadec
hexadecane = 16
alkanes
is based on the number of carbon atoms in the chain and always end with –ane
Alkyl groups
are substituents derived from alkanes
CH3CH2 CH2CH2–
butyl
CH3CH2CH2–
propyl
CH3CH2–
ethyl
CH3–
methyl
Alkyl Groups
are named according to their number of carbons, the same way the naming of alkane is done, but end with –yl instead of –ane.
Branched Alkyl groups
isopropyl, isobutyl, sec-butyl, tert-butyl, and neopentyl
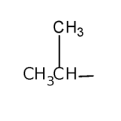
isopropyl
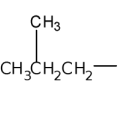
isobutyl
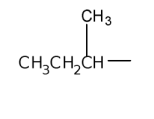
sec-butyl
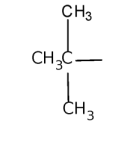
tert-butyl
neopentyl
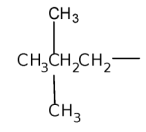
Hyphens
to separate locants and the substituent name
cycloalkanes
are based according to the number of carbon atoms in the cyclic structure
an alkyl group will be obtained
If one hydrogen is removed from the end of the chain of an alkane
cyclopropane
3
cyclobutane
4
cyclopentane
5
cyclohexane
6
cycloheptane
7
cyclooctane
8
alkyl-substituted cycloalkane
If the number of carbons atoms in the ring is the same or greater than that of the alkyl group, the compound is named as an
cycloalkyl-substituted alkane
If the number of carbon in the largest alkyl group attached to the ring is greater than that of the ring, the compound is named as a