Unit 4 Orbitals
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Angular nodes
Straight cut across, touches the center
Radial node
Circular cut
sp3 hybrid
Use for tetrahedral molecules (109.5 degrees)
sp2 hybrid
use for trigonal molecules (120 degrees)
sp hybrid
use for linear molecules (180 degrees)
Hybrid structure/drawing
pointy shaded bits point towards the middle, bulbous unshaded parts on the outskirts
Amplitude determines…
total amount of energy in a wave. Bigger amplitude = more power
Wavelength of light determines
How much energy an individual molecule is able to grab out of light at one time
Light is quantized into fixed-sized pieces called
photons
Ephoton =
hc/wavelength = hv (frequency)
Intensity of light
The number of photons
More nodes means
shorter wavelength, more energy
To eject more electrons from a sample…
Make the light brighter
How to tell which element’s ionization energy is higher? How to tell which molecule’s energy is higher.
Element IE is higher as the radius gets smaller. Molecule IE is higher if its highest energy orbitals are bonding rather than antibonding.
How to tell which molecule donates (HOMO) and which accepts (LUMO) in electron transfer
The donor is the species with the higher-energy HOMO. The acceptor is the species with the lower-energy LUMO.
MO diagram rule
For B, C, & N, pi 2p is lower energy than sigma 2p
For O, F, Ne, sigma 2p is lower energy than pi 2p
Only consider valence electrons when filling it in and writing the orbital types
How to tell if electron lone pair is in a p orbital/delocalized
Leftover p orbitals form delocalized pi bonds in large molecules
If the atom its attached to is sp2 and next to a pi bond or part of a ring.
If electrons can be moved to make a resonance structure, electrons are delocalized.
Higher energy light means
larger energy gap
Lower energy light means
smaller energy gap
Removing a proton can sometimes allow more atomic orbitals to combine into MOs because
A lone pair may be created or become available, and it can participate in conjugation, allowing more MOs to be formed
sp hybridization, how many unhybridized p orbitals remain
2 p orbitals
sp2 hybridization, how many unhybridized p orbitals remain
1 p orbital
sp3 hybridization, how many unhybridized p orbitals remain
0 p orbitals
Find number of photons
Total energy/Energyphoton (Get from plugging wavelength into formula)
If the removed electron comes from an anti-bonding orbital
the bond order will increase by 0.5, and the bond attraction (strength) will increase
If the removed electron comes from a bonding orbital
the bond order will decrease by 0.5, and the bond attraction (strength) will decrease.
weaker bonds are
longer bonds
If highest energy orbitals are antibonding…
easier to ionize than bonding orbitals, lower ionization energy
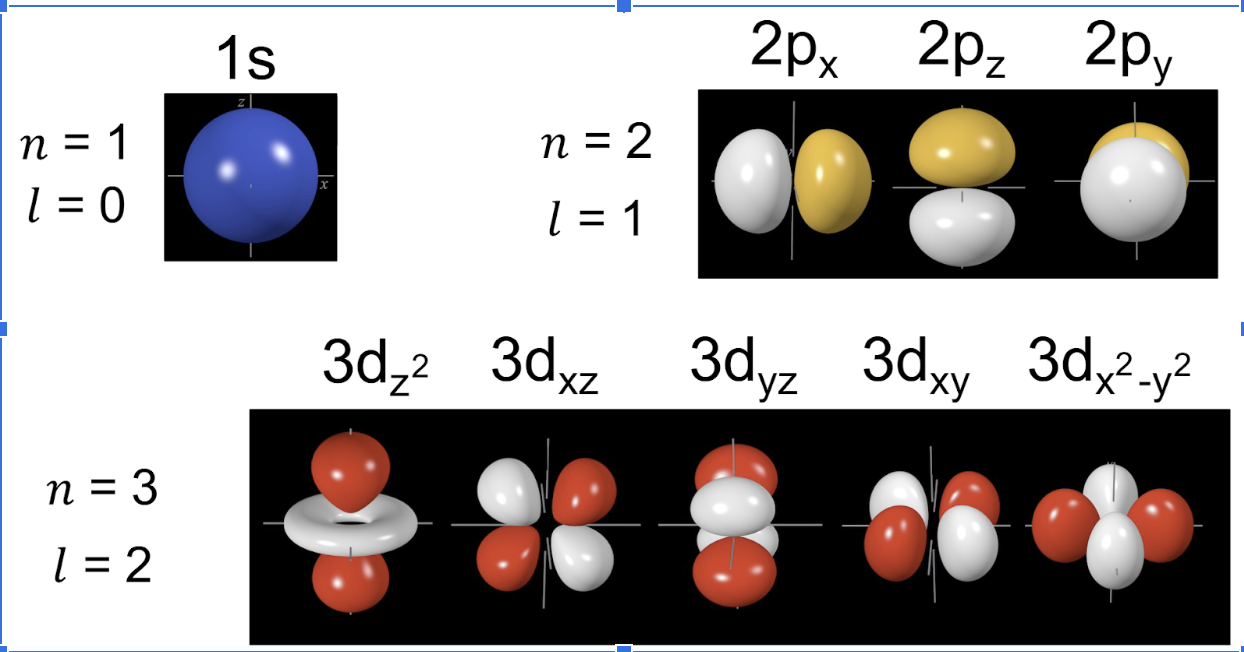
Atomic orbitals
ΔE > 0:
absorption
ΔE < 0:
emission
The more atoms involved in the extended 𝜋 system
the smaller the change in the electron “wavelength” in the HOMO-to-LUMO transition.
Pi bonds always use
pure p orbitals
More protons =
stronger pull, higher IE
Larger radius =
weaker pull, lower IE
More core electrons =
less pull on outer electrons = lower IE
Electrons become excited by
absorbing energy from light/surroundings
Electrons are ejected/ionized when
it gains enough energy from light to overcome the attractive forces holding it to an atom