AP Bio Unit 1 Biochemistry
1/20
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Which of these provides evidence of the common ancestry of all life?
near universality of the genetic code
structure of chloroplasts
structure of the nucleus
structure of cilia
near universality of the genetic code
The best experimental design _____.
includes a control
includes a large sample size and a control, and alters only one condition between the controls and the experimental condition
alters only one condition between the controls and the experimental condition
includes a large sample size for each condition
includes a large sample size and a control, and alters only one condition between the controls and the experimental condition
A controlled experiment _____.
includes one group for which the scientist controls all variables
includes at least two groups, one differing from the other by two or more variables
includes at least two groups, one of which does not receive the experimental treatment
is repeated many times to ensure that the results are accurate
includes at least two groups, one of which does not receive the experimental treatment
In what way are elements in the same column of the periodic table the same? They have the same number of _____.
electrons when neutral
electrons in their valence shells when neutral
electron shells when neutral
protons
electrons in their valence shells when neutral
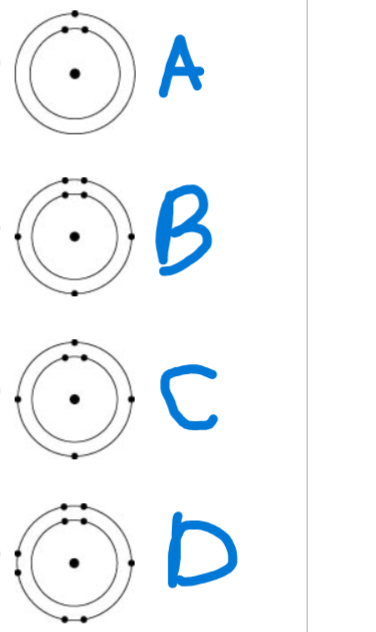
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A
B
C
D
A
A salamander relies on hydrogen bonding to stick to various surfaces. Therefore, a salamander would have the greatest difficulty clinging to a _____.
surface of mostly carbon-nitrogen bonds
surface of mostly carbon-oxygen bonds
slightly damp surface
surface of hydrocarbons
surface of hydrocarbons
What is the maximum number of covalent bonds that an oxygen atom with atomic number 8 can make with hydrogen?
1 covalent bond
6 covalent bonds
4 covalent bonds
2 covalent bonds
2 covalent bonds
A covalent bond is likely to be polar when _____.
the two atoms sharing electrons are the same elements
one of the atoms sharing electrons is more electronegative than the other atom
the two atoms sharing electrons are equally electronegative
carbon is one of the two atoms sharing electrons
one of the atoms sharing electrons is more electronegative than the other atom
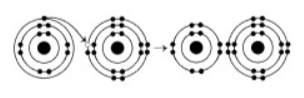
What results from the chemical reaction illustrated above? The reactants have no charge.
a cation with a net charge of +1 and an anion with a net charge of +1
a cation with a net charge of -1 and an anion with a net charge of +1
a cation with a net charge of -1 and an anion with a net charge of -1
a cation with a net charge of +1 and an anion with a net charge of -1
a cation with a net charge of +1 and an anion with a net charge of -1
When are atoms most stable?
when all of the electron orbitals in the valence shell are filled
when all electrons are paired
when they have the maximum number of unpaired electrons
when they have the fewest possible valence electrons
when all of the electron orbitals in the valence shell are filled
When the atoms involved in a covalent bond have the same electronegativity, what type of bond results?
a polar covalent bond
an ionic bond
a hydrogen bond
a nonpolar covalent bond
a nonpolar covalent bond
Which example illustrates a property that emerges at the community level?
Photosynthesis takes place only when pigment molecules are arranged in a specific way in an intact chloroplast.
Metabolic cooperation among many species of prokaryotic cells forms a biofilm that allows bacterial colonies to transport nutrients and wastes. Biofilms may damage industrial equipment or cause tooth decay.
Nitrogen cycling is the process by which nitrogen from the atmosphere and decomposed organic material are converted by soil bacteria to compounds that can be assimilated by plants.
Metabolic cooperation among many species of prokaryotic cells forms a biofilm that allows bacterial colonies to transport nutrients and wastes. Biofilms may damage industrial equipment or cause tooth decay.
Select the most accurate statement about the interaction between a tree and its physical environment.
A tree and its physical environment alter each other.
A tree is affected by its physical environment.
A tree alters its physical environment.
A tree and its physical environment alter each other.
The universal genetic language of DNA is common to virtually all organisms on Earth, however diverse. What is the best explanation for this fact?
The universal nature of the genetic language of DNA is due to coincidence.
The universal genetic language is explained by the chemistry of DNA and proteins.
All living things share a common genetic language of DNA because they share a common ancestry.
All living things share a common genetic language of DNA because they share a common ancestry.
Select the correct statement about the process of scientific inquiry.
If the results of an experiment do not support the hypothesis that is tested, the experiment is badly designed.
It is possible to test hypotheses, such as those involving historical events, without conducting experiments.
The goal of scientific research is to prove the stated hypothesis.
It is possible to test hypotheses, such as those involving historical events, without conducting experiments.
Which of these examples illustrates deductive reasoning?
You come down with the flu after you pretended to be sick so you could skip work to attend a concert. Now you decide that fate always punishes you for bad behavior.
You learned in elementary school that as temperature drops, liquids change into solid form. You are given an unfamiliar liquid and predict that it will become solid if you put it in the freezer.
You and your roommate both drank two cups of coffee before an exam, and both of you got a better than average mark on the exam. You hypothesize that coffee drinking improves academic performance.
You learned in elementary school that as temperature drops, liquids change into solid form. You are given an unfamiliar liquid and predict that it will become solid if you put it in the freezer.
Which statement about weak bonds is correct?
Weak bonds are less important to living things than strong covalent bonds.
Weak chemical bonds form only between atoms of similar electronegativity.
Weak bonds are transient and easily reversible
Weak bonds are transient and easily reversible
Which of the following bonds can form between atoms of equal electronegativity?
Ionic bonds can form between atoms of equal electronegativity.
Van der Waals interactions can form between atoms of equal electronegativity.
Hydrogen bonds can form between atoms of equal electronegativity.
Van der Waals interactions can form between atoms of equal electronegativity.
Which statement about relative potential energy of electrons is correct?
An electron in the 3 p orbital of the third electron shell has more potential energy than an electron in the 2 p orbital of the second electron shell.
An electron in the 2 p orbital of the second electron shell has more potential energy than an electron in the 3 p orbital of the third electron shell.
An electron in the 2 p orbital of the second electron shell has more potential energy than an electron in the 2 s orbital of the second electron shell.
An electron in the 3 p orbital of the third electron shell has more potential energy than an electron in the 2 p orbital of the second electron shell.
What happens when two atoms form a chemical bond?
Two atoms fuse together to form a chemical bond.
A chemical bond forms when two atoms transfer or share outer electrons to complete their outer shells.
A chemical bond forms when two atoms transfer or share protons to achieve a stable nucleus.
A chemical bond forms when two atoms transfer or share outer electrons to complete their outer shells.
What does the term electron orbital describe?
An electron orbital describes the exact distance of an electron from the nucleus.
An electron orbital describes the orbit of an electron around the nucleus.
An electron orbital describes a three-dimensional space where an electron can be found 90% of the time.
An electron orbital describes a three-dimensional space where an electron can be found 90% of the time.