Macromolecules: polymers, proteins and carbohydrates
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What are the 12 principles of green chemistry?
1. Prevent waste
2. Maximise atom economy
3. Design less hazardous chemical
4. Design safer chemicals and products
5. Use safer solvents and reaction conditions
6. Increase energy efficiency
7. Use renewable reactants
8. Avoid using chemical derivatives
9. Use catalysts
10. Design chemicals that will degrade
11. Prevent pollution
12. Minimise the potential of chemical accidents
Atom economy formula:
m(products)/m(reactants) × 100
e.g extraction of copper from copper oxide
2CuO(s) + C(s) → 2Cu(s) + CO2(g)
ae% = 2(63.546) 2(63.546 + 16) + 12 × 100 ae% = 127.092
171.092 × 100 ae% = 127.092 171.092 × 100 = 74.28%
top-down vs bottom-up approaches to molecular manufacturing
Top Down: big molecules into smaller/more useful ones. Uses lots of material, energy and waste.
Bottom-up: building molecules 1 bit at a time. More difficult due to size but less waste and energy.
Three methods of molecular manufacturing:
Orientation effect (positioning)
manipulating structures (add/remove parts of molecules)
Use of a protecting group (adding a bodyguard)
Explain how either synthetic proteins, carbon nanotubes, nanorobots and chemical sensors in medicine is an example of a specialised product produced by molecular manufacturing.
Molecular manufacturing utilises precise control at the atomic or molecular level to build structures for medicine, creating synthetic proteins, carbon nanotubes, nanorobots, and chemical sensors as highly specialised tools.
e.g synthetic proteins are engineered for specific functions in the body,
Carbon nanotubes are used as drug delivery vehicles or biosensor components due to their unique electrical and physical properties
nanorobots are designed for targeted therapy and diagnostic tasks
Chemical sensors leverage nanomaterials to detect disease biomarkers with high sensitivity.
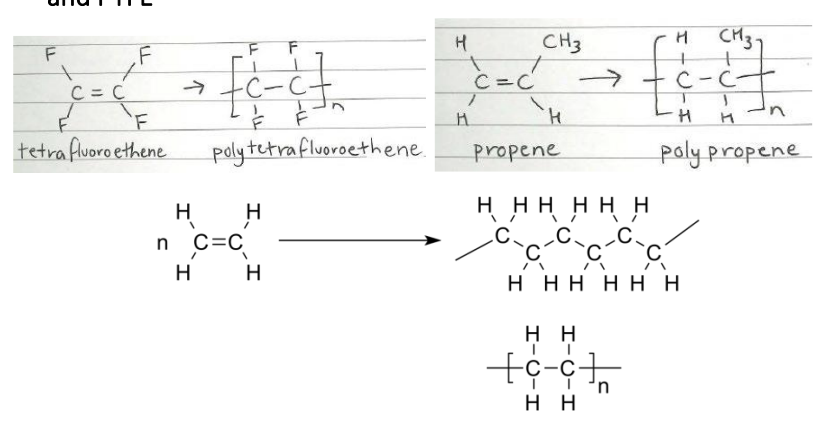
Using equations, compare the formation of LDPE, HDPE, polypropene and PTFE
advantages and disadvantages of polymer use, including strength, density, lack of reactivity, use of natural resources and biodegradability
Disadvantages: Considering polymers are in synthetic clothing fibres, polyethene cups, fibre glass, paints, plastic bags, they have disadvantages. Pollution is a significant disadvantage to polymer use, despite its convenience - i.e. single-use plastic bags. Most polymers are non-biodegradable.
Advantages: Are more resistant. Due to van der Waals attractions and entanglements, the strength of the polymer increases as the chain gets longer. Density - Are flexible (sporting equipment). Have lack of reactivity because they are resistant to corrosion.
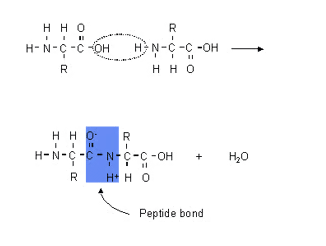
Describe the condensation reaction of 2-amino acids to form polypeptides (involving up to three amino acids)
Polypeptide chains are made by joining amino acid monomers together through peptide bonds. Peptide bonds between amino acids are formed in a reaction between the carboxyl group of one amino acid and the amino group of another. This reaction is condensation reaction because it produces a molecule of water
Describe the condensation reaction of monosaccharides to form disaccharides (lactose, maltose and sucrose)
Lactose: Is formed by glycosidic linkage in a condensation reaction. Is formed when two monosaccharides (glucose and galactose) combine.
Maltose: Is formed by two units of glucose joined with a glycosidic bond. Specifically, alpha glucose linkage is made.
Sucrose: is formed when a monomer of glucose and a monomer of fructose are joined in a dehydration reaction to form a glycosidic bond.
Describe the condensation reaction of polysaccharides (starch, glycogen and cellulose)
Starch: Is a mixture of two polysaccharides - amylose and amylopectin. Amylose is straight chained, with a a(1-4) glycosidic linkage whereas amylopectin has a (1-6) glycosidic linkage and is branched.
Glycogen: Is formed by the condensation reaction from alpha glucose. Is branched and is made of linear chains of glucose residues.
Cellulose: is formed by the condensation of β-glucose. Is made up of glucose monomers that are linked by β 1-4 glycosidic bond and is packed tightly
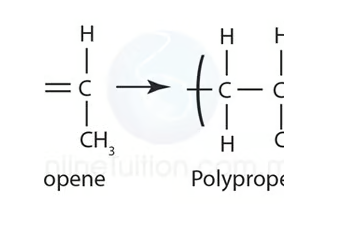
Describe, using equations, how addition polymers can be produced from their monomers including polyethene (LDPE and HDPE), polypropene and polytetrafluorethene. (Polypropene)
Polypropene: Propene bond breaks, causing (picture) due to addition reaction.
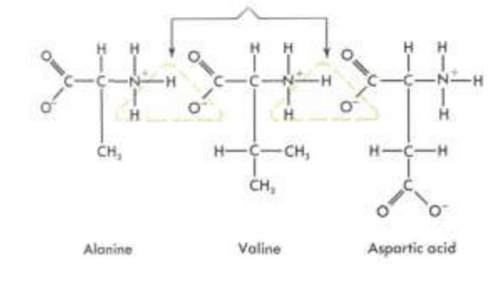
how a polypeptide with 3 amino acids is formed
To form polypeptides and proteins, amino acids are joined together by peptide bonds, in which the amino or NH2 of one amino acid bonds to the carboxyl (acid) or COOH group of another amino acid.
How are how polysaccharides are formed
Polysaccharides are formed by the combination of a large number of monosaccharides through the glycosidic bond
Identify which monosaccharides react to form lactose, maltose, sucrose, starch, glycogen and cellulose:
Galactose + Glucose → Lactose + Water
Glucose + Glucose → Maltose + Water
Galactose + Fructose → Sucrose + Water
Glucose (x300+) (Branching) → Starch
Glucose (x300+) (Linear) → Cellulose
Glucose (x8) → Glycogen