Group 2 and Group 1 metals
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Group 2 + water?
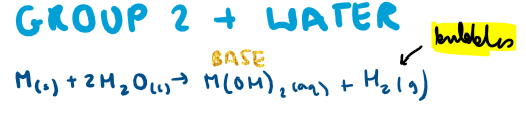
What is the trend down the group for reactivity with water?
Reactivity increases down the group with water -> larger atomic radii down the group -> electron further from nucleus + more shielding -> electron easier to remove -> hence more reactive
Which group 2 metals cannot react with water and why?

What is the reaction of magnesium with water?
Water MUST have gas sign (steam)

Group 2 + oxygen?
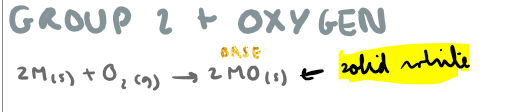
Group 2 oxide + water?

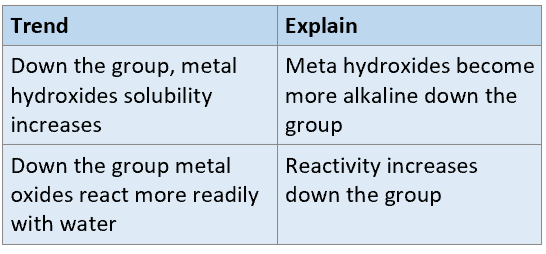
What are the trends down the group for group 2 oxides + water reactions?
Group 2 oxide + acid?
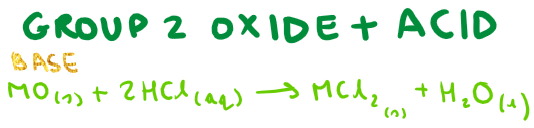
Group 2 hydroxide + acid?
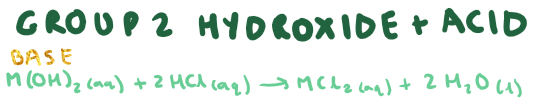
What is the type of reaction that is between a group 2 oxide and acid + group 2 hydroxide and acid?
Neutralisation
What is the trend of solubility for both G1 and G2 sulphates?
Solubility decreases down the group
What is the trend of solubility for both G1 and G2 hydroxides?
Solubility increases down the group
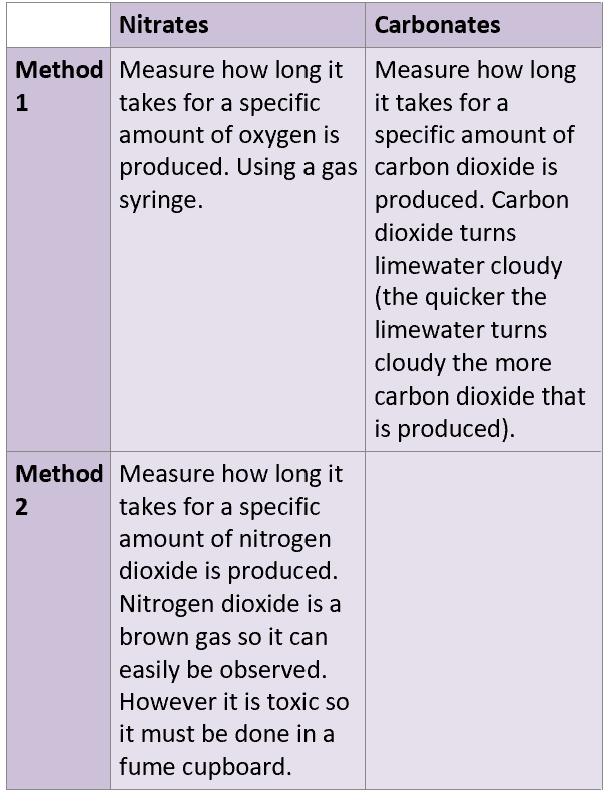
G2 carbonate decomposition? (what type of decomposition is this?")

G2 nitrate decomposition? (state any observations)
Brown toxic fumes from nitrogen dioxide
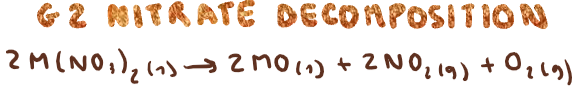
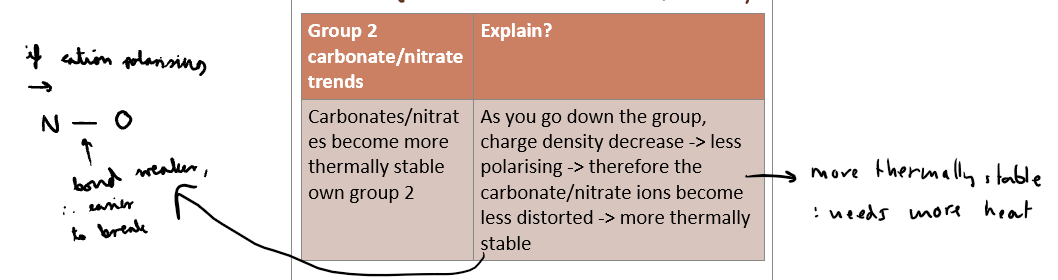
What is the trend for thermal stability for G2 carbonates and nitrates?
G1 carbonate decomposition?
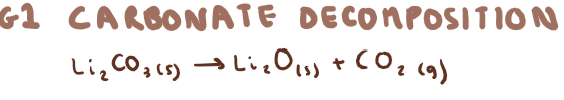
What are exceptions to G1 carbonate decomposition and why?

G1 nitrate decomposition? (state any observations)
Brown toxic fumes from nitrogen dioxide
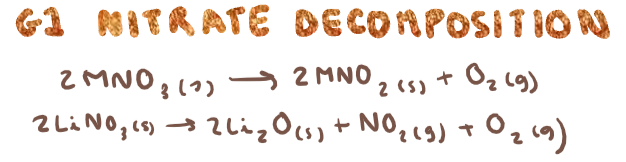
What is trend for thermal stability of G1 nitrates and carbonates?
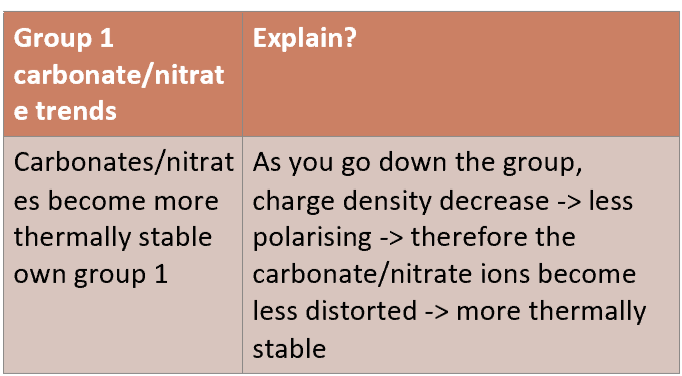
How do you test for thermal stability of G1 and G2 carbonates and nitrates?