pericyclic reactions
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
what is a conjugated diene
A conjugated diene is a hydrocarbon containing two double bonds that are separated by a single bond, allowing for resonance and increased stability. This structure enables the diene to participate effectively in pericyclic reactions.
when is there an EN in a diels alder reaction
if there is a chiral center or a trans dienophile
cis dienophiles will be meso compounds UNLESS THEY HAVE 2 DIFF substit
also must be sp3 carbon to. be dashed or wedged
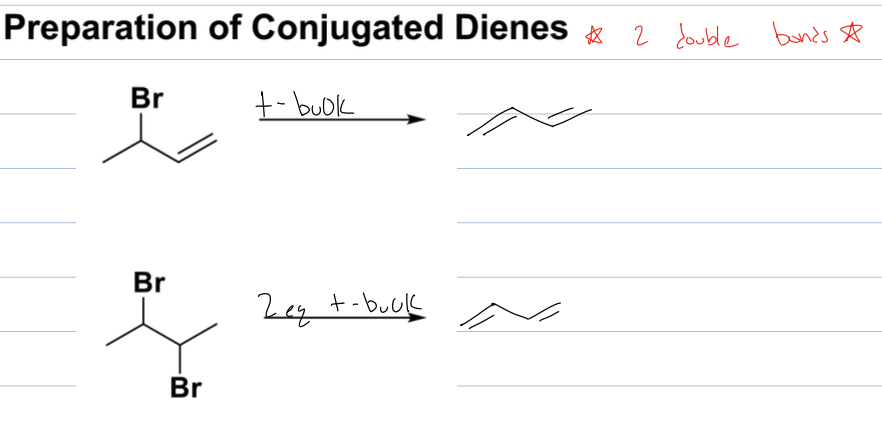
what is rxn to get a conjugated diene
elimination reaction of a substituted alkene with a bulky base
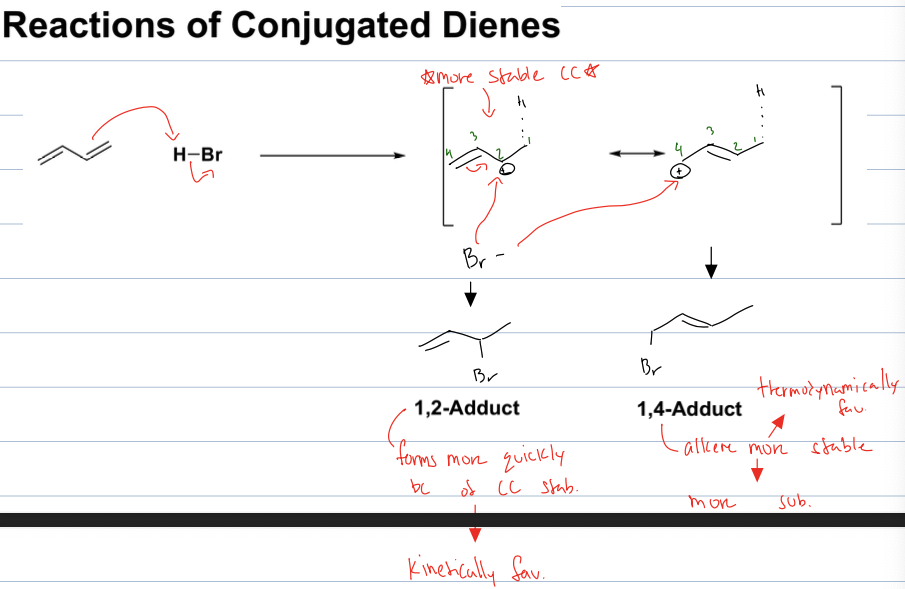
what rxn with a conjugated diene and acid halide
the double bond will attack acid halide and deprotonate leaving a halide ion and a carbocation intermediate
depending on the diene a more stable CC can form leaving a kinetically favored product and a thermodynamically favored product when the halide ion binds to the CC. the halide at more stable CC will be kinetically favored bc it will form faster. but the halid eat the more unstable CC will be thermodynamiclaly favored because produce is a more stable alkene due to it being more substituted.
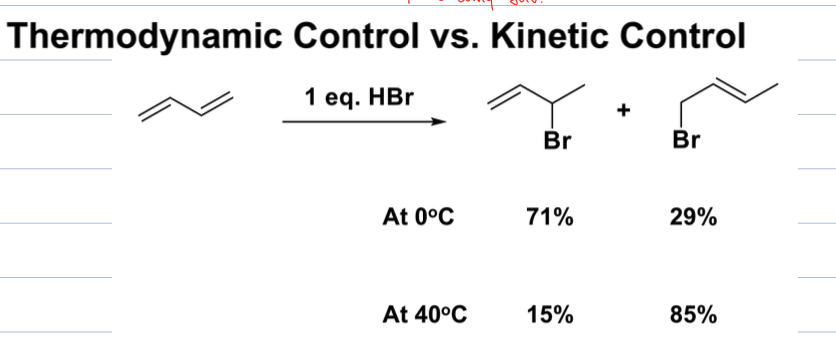
what is thermodynamic control vs kinetic control of conjugated dienes
Thermodynamic control refers to the selectivity of reactions that favor the formation of the most stable product, while kinetic control favors the formation of products that can form more rapidly, regardless of their stability. which product is produced can be determined by the temerature, at higher temps the thermodynamically preferred produc(less sub)t is formed by overcomig Ea and at lower temps more sub or kinetically favored product is formed
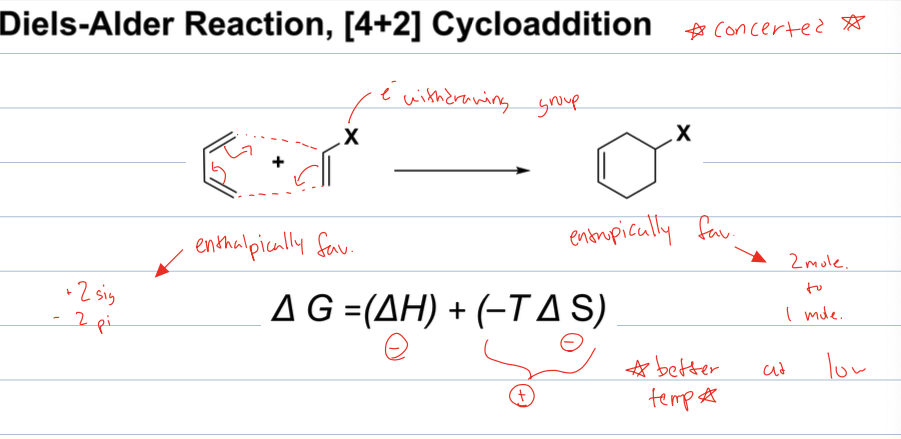
what is rxn with diene and dienophile
The reaction between a diene and a dienophile, commonly called a Diels-Alder reaction, forms a six-membered ring through a concerted cycloaddition.
what is the stereospecificity of diels alder rxn
The stereospecificity of the Diels-Alder reaction refers to the preservation of the stereochemistry of the diene and dienophile in the product. This means that the configuration of substituents on the reactants will determine the configuration of the substituents in the resulting six-membered ring. if the dienophile is cis the product will be cis and meso if it is trans they will be trans and have an en (reacemic mixture)
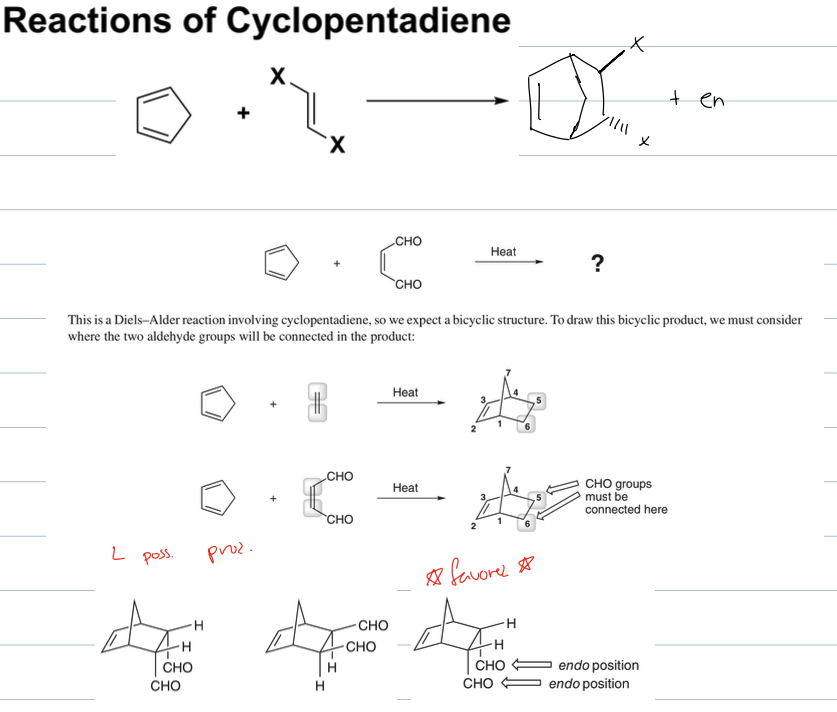
what is rxn if diene is cyclopentadine and a dienophile
The reaction of cyclopentadiene with a dienophile results in a Diels-Alder reaction, forming a bridged bicyclic structure that is often highly stable due to its unique ring system.
what is the regioselectivity of diels alder rxn
draw resonance structure of diene and dienophile, then line up part. pos and part. nrg. ends to complete rxn.