Chemistry - Topic 2: Managing Chemical Processes
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
Rate of reaction
expressed as rate of formation of a product or the rate of consumption of a reactant.
can be measured as the change per unit time in concentration, mass or amount of product or reactant respectively
Average rate of reaction
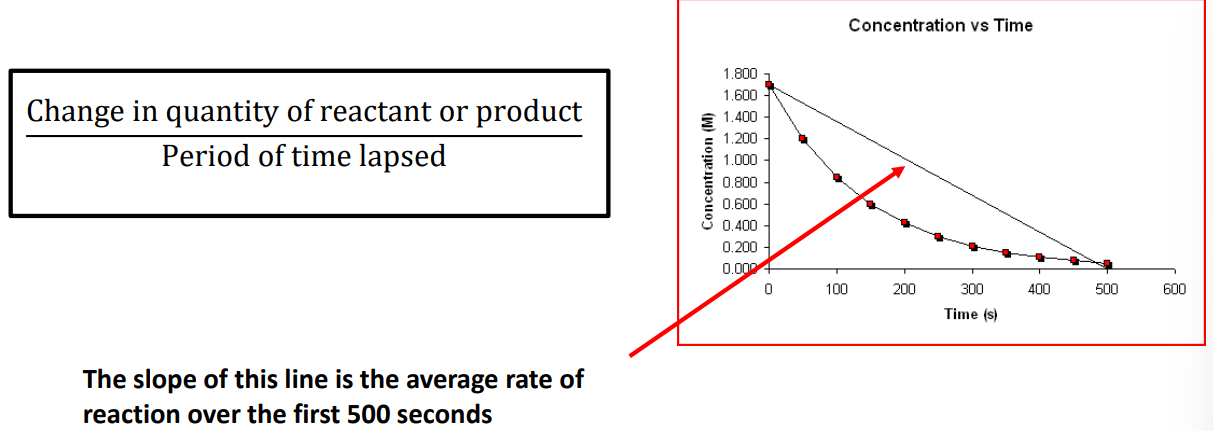
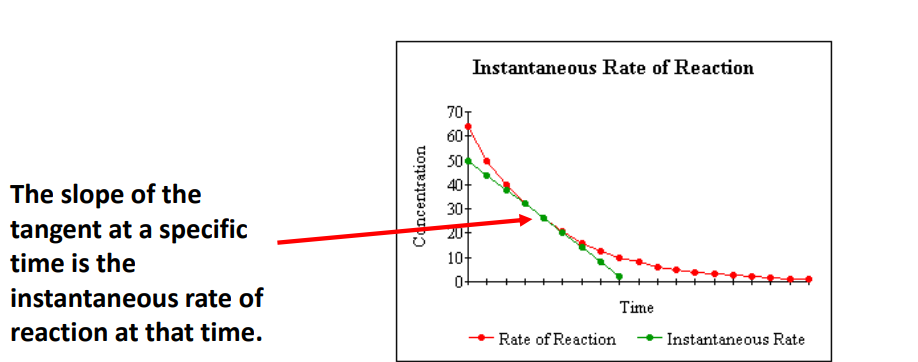
Instantaneous rate of reaction
describes the rate at a particular instant of time.
the gradient of the quantity of reactant (or product) vs time graph at that particular time.
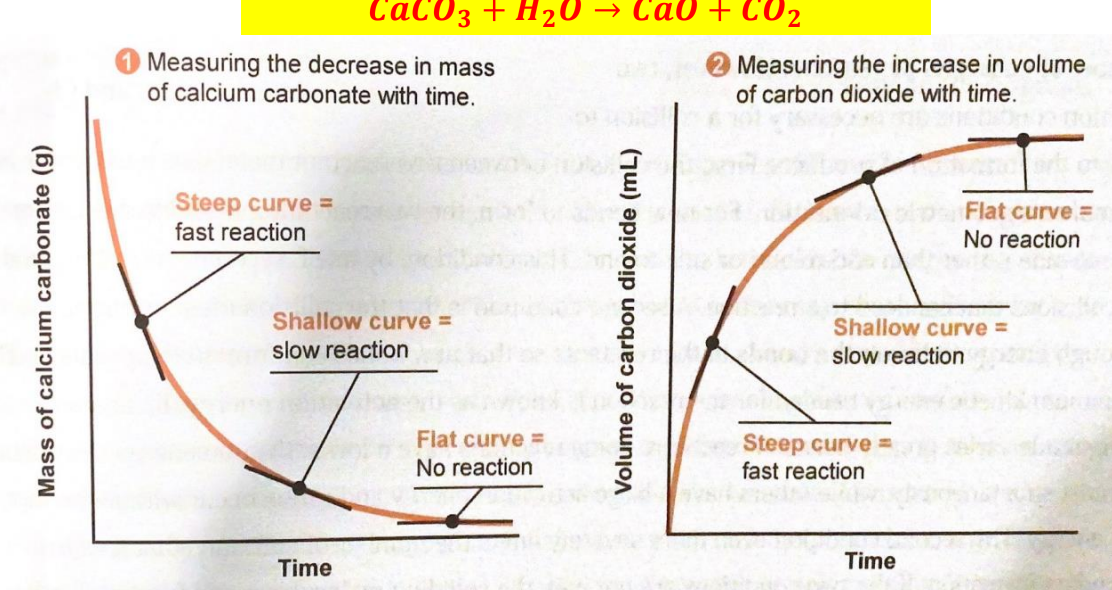
Measuring decrease in mass with time and increase in volume with time (reading graphs and determining slopes)
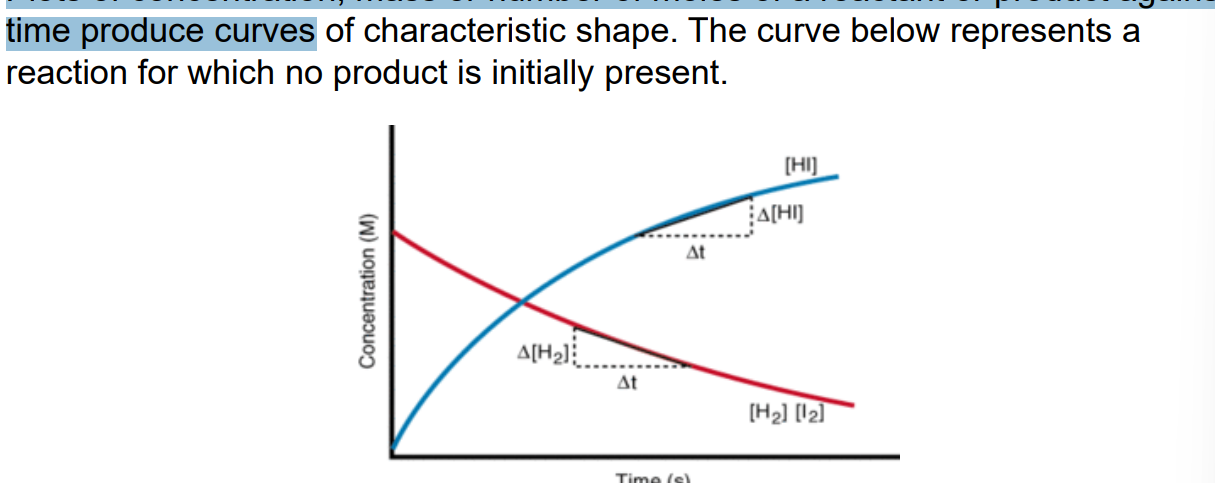
quantity-time graphs
Plots of concentration, mass or number of moles of a reactant or product against time produce curves
Collision theory
• In order for a reaction to occur between atoms, ions or molecules, they must first collide.
• Collisions which result in a reaction are called productive collisions
• Collisions which do not result in a reaction are nonproductive
Productive collisions
reactants must:
• Have at least the minimum kinetic energy necessary for breaking bonds and forming new ones (THIS IS THE ACTIVATION ENERGY)
•Be correctly orientated at the time of collision
Reaction Rate depends on
• The magnitude of the activation energy barrier
• The frequency of collisions
• The energy of collisions relative to the activation energy
• The orientation of the colliding particles
Factors affecting reaction rate
•Concentration of reactants
•Pressure
•Temperature
•Surface area of solid reactants
•Catalysts
• Intensity of Incident light
Concentration
Increase concentration → increase number of reactant molecules per volume →increase the frequency of collisions between reacting particles → increase the probability of successful collisions → increase the rate of reaction.
pressure
AFFECTS GASES ONLY
Higher pressure→ reduce volume →Higher number of reactant molecules per volume →Higher frequency of collisions between reacting particles → higher probability of successful collisions → higher reaction rate.
Surface are of solid reactants
Increase surface area to volume ratio → increase the number of surfaces over which an effective collision can occur→ increase the frequency of successful collisions→ increase reaction rate.
Temperature
Higher temperature → higher kinetic energy, therefore more collisions have enough energy to overcome activation energy barrier → higher probability of successful collisions → higher reaction rate
Catalyst
Use of catalyst→ lower the activation →higher probability of successful collisions → increase reaction rate.
Intensity of Incident Light (e.g.)
Increasing intensity of incident light will increase the rate of photochemical reactions.
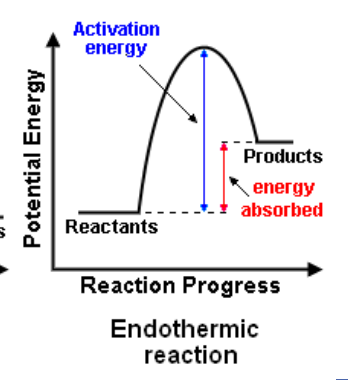
Energy profile diagrams (endothermic)
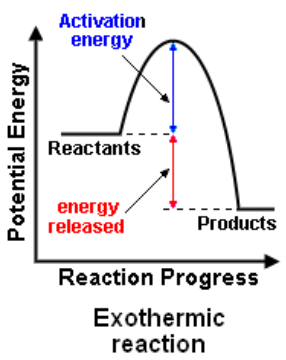
nergy profile diagrams (exothermic)
Energy and reactions
• Chemical reactions usually result in absorption or release of energy in the form of heat, light, electrical or sound energy.
• For reactions which release or absorb heat, the quantity of heat energy released or absorbed when specified amounts of substances react is called the heat of reaction.
• For reactions carried out at constant pressure, the heat of reaction is called the enthalpy change for the reaction.
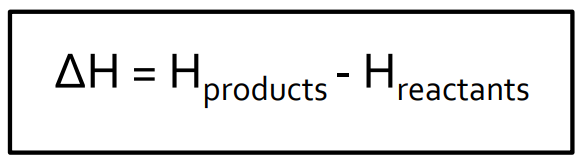
Enthalpy
The enthalpy change for a reaction is the difference between the enthalpies of the reactants and products.
Units: Kilojoules per mole (KJmol−1 )
Joules per mole (J mol−1 )
Kilojoules per gram (KJ g −1 )
→ KJmol−1 can be converted to KJ g −1 by dividing by the molar mass
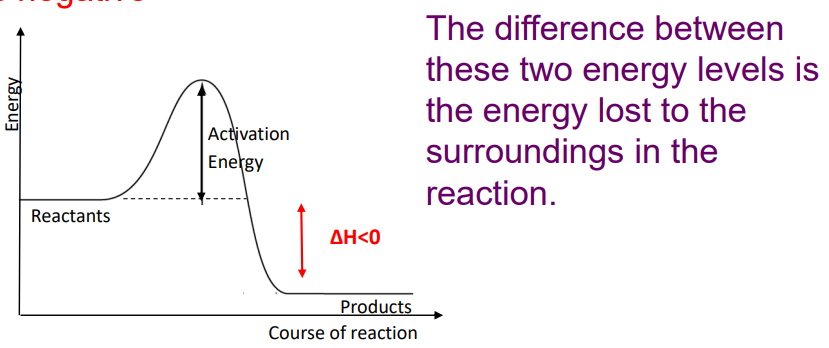
Exothermic reactions
EXOthermic reactions RELEASE heat energy to the surroundings
reactants have a higher enthalpy (heat content) than the products
The temperature of the surroundings INCREASES
e.g. combustion reactions, respiration
Enthalpy change (ΔH) is negative
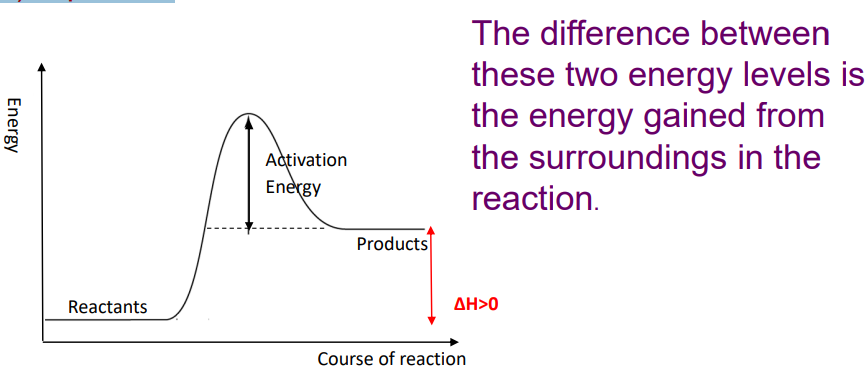
Endothermic reactions
ENDOthermic reactions ABSORB heat from the surroundings
temperature of the surroundings DECREASES
e.g. melting, evaporation
the products have a higher enthalpy (heat content) than the reactants.
Enthalpy change (ΔH) is positive
Equilibrium
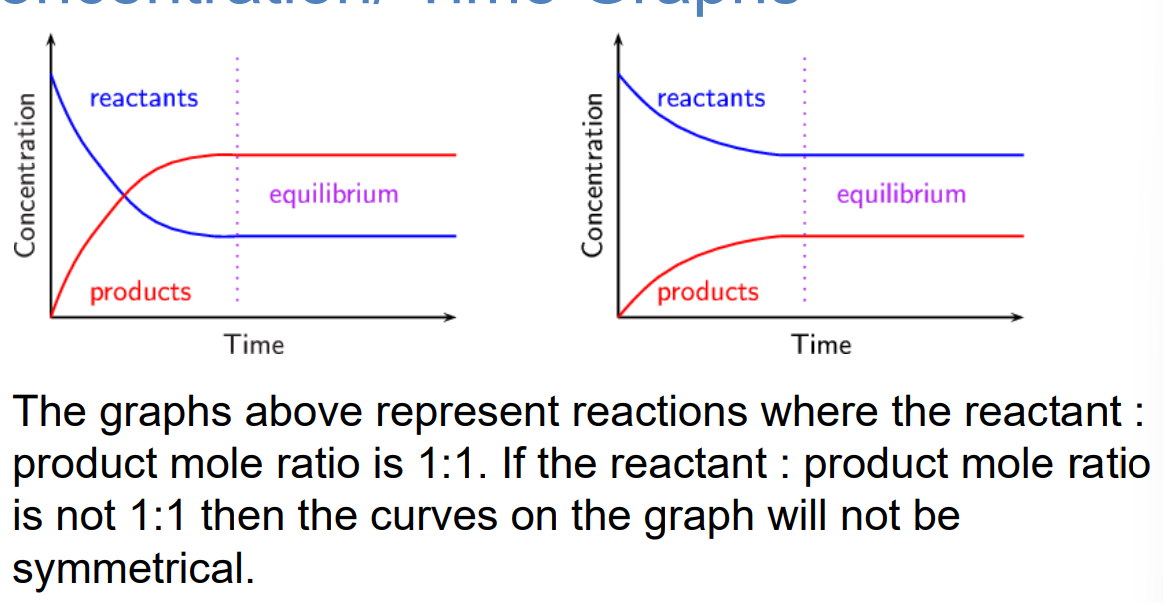
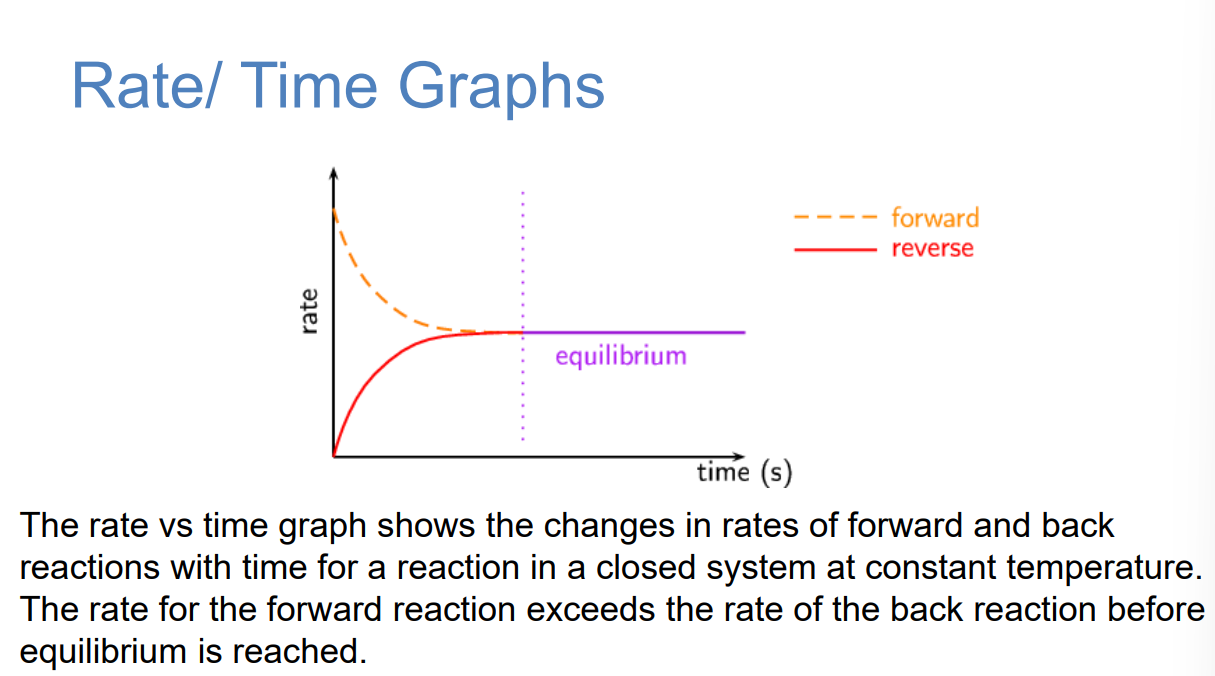
Equilibrium is a dynamic state in which the forward and back reactions are ongoing, and the rate of the forward reaction is equal to the rate of the back reaction. At equilibrium, the net concentrations of the reactants and products in the reaction mixture are each constant with time.
Concentration/ Time Graphs
Rate/ Time Graphs
Equilibrium Constant
describe the yield of the products relative to the quantities of reactants.
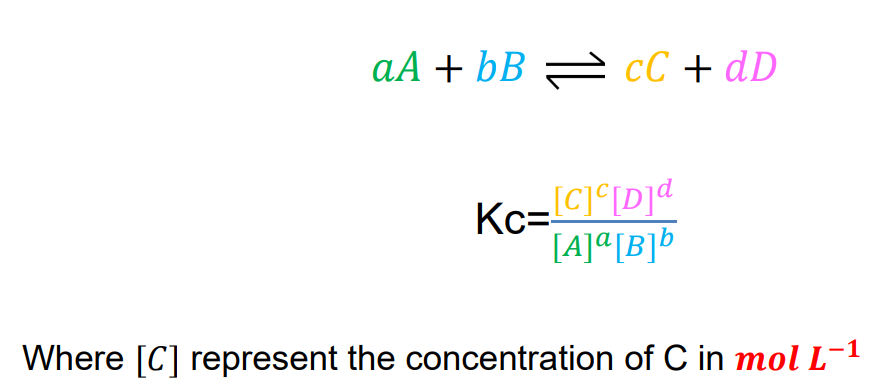
Large Kc
greater than 10
indicates high yield of products at the equilibrium position
reaction has proceeded to a large extent to the right by the time the equilibrium position is reached.
Small Kc
less than 0.1 indicates:
low yield of products at the equilibrium position
That the reaction has proceeded to a small extent to the right by the time the equilibrium position is reached.
ICE Table
Initial concentration, change in concentration (determined with molar ratio, reactants are always negative), equllibrium concentration
Le Chatelier’s Principle
If an external change is made to the reaction conditions of a system at equilibrium so that it is no longer at equilibrium, a net reaction will occur (if possible) in the directiont hat counteracts the change; the internal change will oppose the external change.
Changes to concentration (LCP)
Increasing the concentration of a reactant will shift equilibrium to the right (in the direction of consumption of the reactant).
according to LCP, the system will try to counteract the change of increased concentration by increasing othe reactant concentration. The equilibtium then shifts to the right, which reduces the reactant concentration.
Concentration changes on reaction pathway diagram
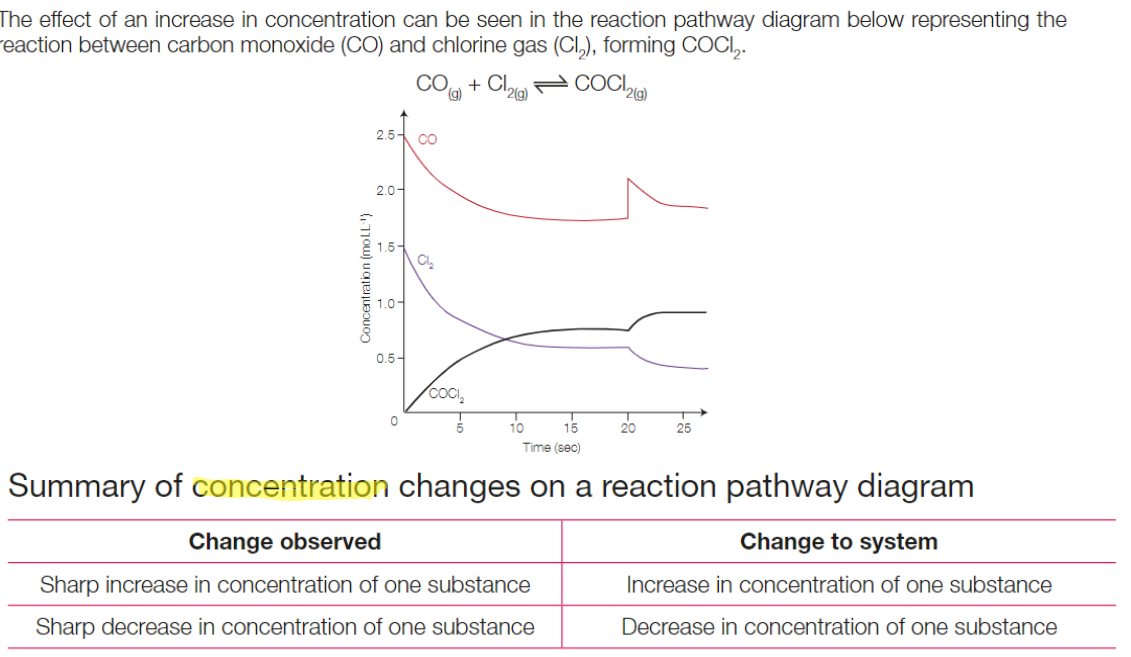
Changes to pressure (LCP)
Increasing the pressure of the system will decrease the volume of the reaction vessel so equilibrium is shifted to produce a smaller number of molecules in the gas phase.
Decreasing the pressure of the system will increase the volume of the reaction vessel so equilibrium is shifted to produce a greater number of gas molecules.
According to LCP, the system tries to counteract the effect of increased temperature by shifting the equilibrium towards the size with fewer moles. Therefore, shifting equillibrium to the (fewer moles; left/ right), and favouring forwards/ backwards reaction.
Summary of pressure changes on reaction pathway diagram
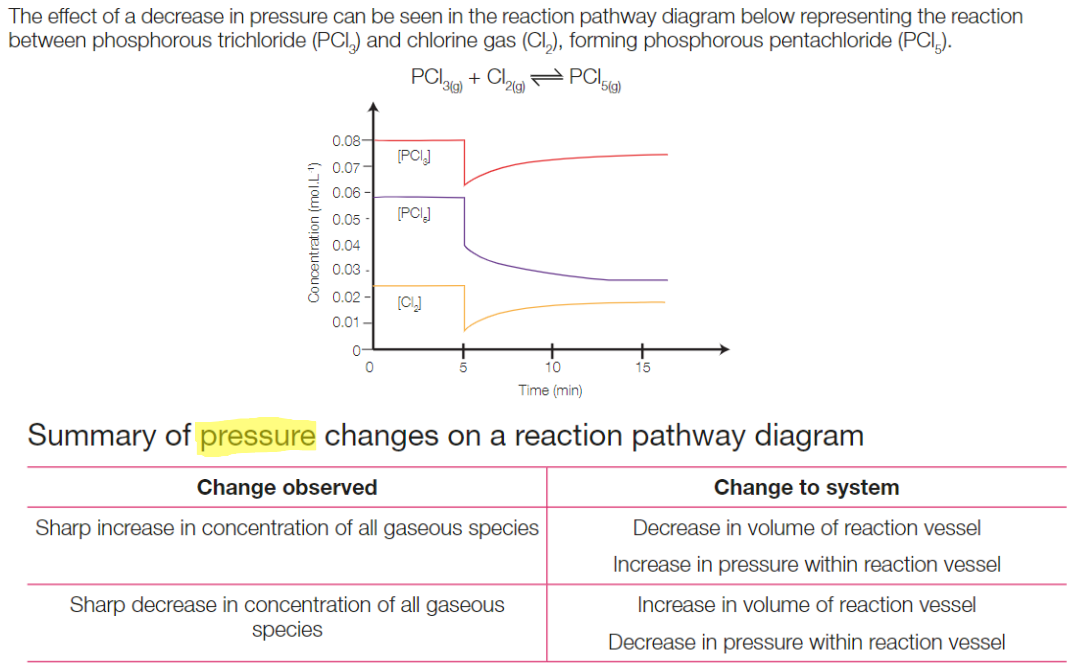
Changes to temperature
According to LCP, system tries to counteract increase in temperature by shifting the equilibrium to the left, favouring
Summary of temperature changes on reaction pathway diagram
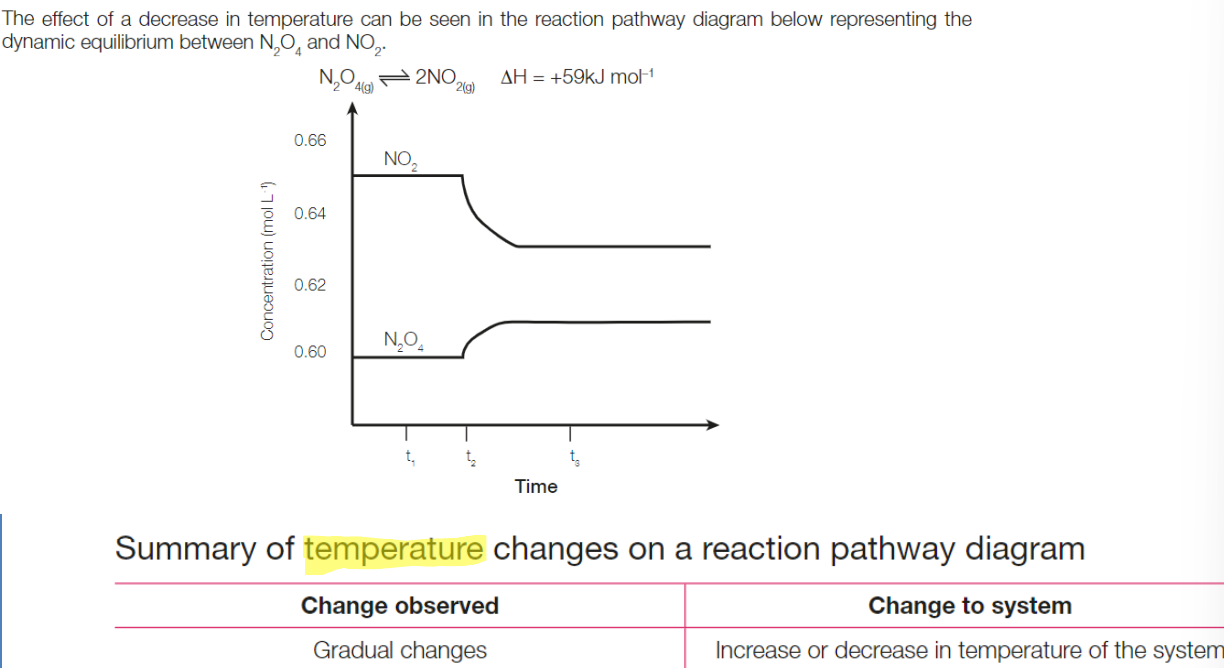
Use of Catalyst
A catalyst will speed up both the forward and back reactions, so there will not be an uneven shift in either direction at equilibrium.
The catalyst will just help to reach the point of equilibrium sooner.
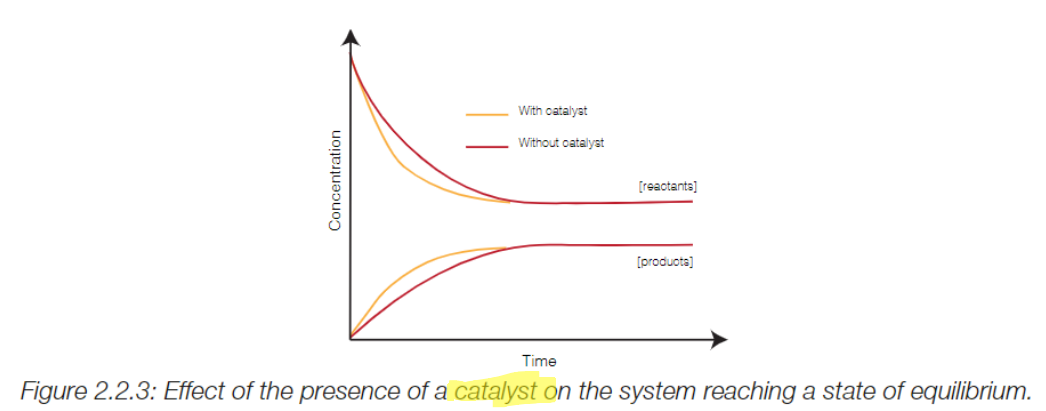
Colorimeter
Measures the concentration of a solution based on its color intensity. It works by passing light through a sample and measuring how much light is absorbed.
Components:
Light Source – Emits a specific wavelength of light.
Filters – Selects the appropriate wavelength for measurement.
Sample Holder (Cuvette) – Holds the solution being tested.
Detector (Photocell/Photoresistor) – Measures the amount of light passing through the sample.
Display/Output – Shows the absorbance or transmittance values.
Working Principle
Light is directed through a filter to obtain a specific wavelength.
The filtered light passes through the sample solution.
The detector measures the amount of absorbed or transmitted light.
The readings are analyzed to determine the concentration of the solution using Beer-Lambert’s Law, which relates absorbance to concentration.