Chapter 14 - Oxidation and Reduction
1/29
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
oxidation in terms of electron transfer
loses ie. if a substance loses electron(s), it is oxidised
reduction in terms of electron transfer
gains ie. if a substance gains electron(s), it is reduced
Note: Reactions in which one substance is oxidised and one substance is reduced are known as redox reactions
oxidising agent
is a substance that brings about oxidation in other substances (goes through reduction)
i.e. causes another substance to lose electrons – therefore the oxidising agent is itself reduced or gains electrons
reducing agent
is a substance that brings about reduction in other substances (goes through oxidation)
i.e. causes another substance to gain electrons – therefore the reducing agent is itself oxidised or loses electrons
oxidation in terms of oxidation number
increases
Show in terms of electron transfer that the rusting of iron is a redox reaction
• Rusting involves the reaction of iron with oxygen to form an oxide of iron
4Fe + 3O2 → 2Fe2O3
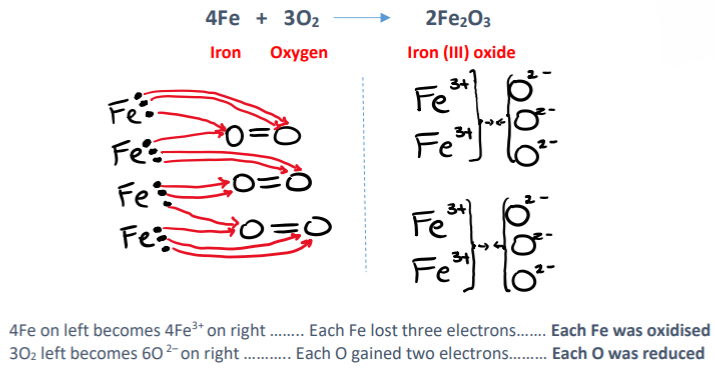
Give an example of an oxidising agent used in swimming pools
• Chlorine (Cl2) is added to swimming pools to disinfect them, it reacts with the water to form free chlorine species; hypochlorous acid (HOCl) and the hypochlorite ion (OCl–) which oxidise and kill micro-organisms
Explain how oxidising agents and reducing agents are used in different bleaches
Oxidising agent: Sodium hypochlorite (NaOCl) is used in household bleaches
Example: Domestos for removing stains from clothes and acting as a disinfectant
Reducing agent: Sulfur dioxide (SO2) is used to reduce and bleach the pigment in wood in the manufacture of paper
Note: In most reactions it can be difficult to tell what is oxidised and reduced/what is the oxidising agent and reducing agent…… we use oxidation numbers
Define oxidation number
• Oxidation number is the charge an atom has or appears to have when electrons are distributed according to certain rules
Rules of oxidation numbers
1. In any neutral compound/molecule the sum of the oxidation numbers must equal 0
2. In any complex ion, the sum of all the oxidation numbers must equal the charge on the ion
3. A free element (An element not bonded to another element or bonded to itself) always has an oxidation number of 0
4. The oxidation number of an ion is equal to the charge on the ion
5. Oxygen always has an oxidation number of -2 in its compounds
6. Hydrogen always has an oxidation number of + 1 in its compounds
7. Fluorine always has an oxidation number of - 1 in its compounds
8. Group 1 elements always have an oxidation number of + 1 and Group 2 elements always have an oxidation number of + 2 in their compounds
9. In the case of ionic compounds, an element’s oxidation number is equal to the charge on its ion
Exceptions to rules of oxidation numbers

How does the oxidation number of the oxidising agent change during a redox reaction?
• The oxidising agent is itself reduced……. oxidation number decreases
How does the oxidation number of the reducing agent change during a redox reaction
• The reducing agent is itself oxidised……. oxidation number increases
Important: When asked to identify oxidising agents and reducing agents, the entire compound is named (unless the question specifically says ‘element’ like 2020! :))
Balancing redox equations using oxidation numbers
Note: Redox reactions typically do not show every element involved and just shows the relevant ions; therefore they cannot be just balanced by inspection, oxidation numbers need to be used
Step 1: Assign oxidation numbers to all elements in the equation
Step 2: Focus on which elements changed in oxidation number
Step 3: Write out how many electrons EACH ATOM of the element reduced gains and how many electrons EACH ATOM of the element oxidised loses
Step 4: Balance the number of electrons lost and the number of electrons gained by multiplying the substance oxidised and the substance reduced by the lowest factors possible
Step 5: Balance the remaining equation as normal by inspection
(Leave oxygen and hydrogen atoms till last if involved)
reduction in terms of oxidation numbers
decreases
elements on their own
0
ions
same as charge
charge of all elements in a compound
0
oxygen
-2
expections: h202, na202 = -1 and of2 = +2
hydrogen
+1
exceptions are the metal hyrbids NaH = -1
halogens
-1 except when joined to a more electronegative element
alkali metals
+1
alkaline earth metals
+2
The electrochemical series
• All metals lose electrons (are oxidised) when they react
• The electrochemical series is a table of metals arranged in order of how easily they lose
electrons (are oxidised) /their reducing abilities/how reactive they are
• The higher up the metal is the more easily it will be oxidised/reduce other substances
Potassium (K)
Sodium (Na)
Calcium (Ca)
Magnesium (Mg)
Aluminium (Al)
Zinc (Zn)
Iron (Fe)
Lead (Pb)
Hydrogen (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
• A metal higher up in the series will reduce a metal lower down and displace it from a compound
Write a balanced equation for the reaction that occurs when magnesium is placed in copper II sulfate solution. Explain this reaction and describe what is observed
• Magnesium is higher than copper in electrochemical series so has a higher reducing ability. Magnesium reduces copper (causes it to gain electrons) and displaces copper from the compound copper (II) sulfate.
• Magnesium sulfate and copper are formed
Observe: - Blue colour of copper (II) sulfate becomes colourless
- A brown precipitate of copper metal forms
What is observed when copper is placed in dilute sulfuric acid. Explain this observation
• Copper is lower than hydrogen in the electrochemical series and will not reduce and displace it from a compound.
Observe: No reaction
Give an application of knowledge of the electrochemical series
• Scrap iron (Iron II) can be used to reduce and displace a more useful but less reactive metal such as copper from a compound
• Copper is extremely useful in the electronics industry Example: For electrical wires
Give an application of knowledge of the electrochemical series
• Scrap iron (Iron II) can be used to reduce and displace a more useful but less reactive metal such as copper from a compound
• Copper is extremely useful in the electronics industry Example: For electrical wires
Which metals from the electrochemical series are found free in nature? Why?
• Lead (Pb), Copper (Cu), Silver (Ag), Gold (Au)
• These metals are at the bottom of the electrochemical series and will not oxidise easily i.e. are very unreactive
Note: Metals at the top of the electrochemical series are very reactive and are only found in compounds (not free in nature)
Why are halogens good oxidising agents?
• Halogens (Group VII elements) usually act as oxidising agents due to their high electronegativity values – they have a strong attraction for electrons and tend to remove electrons from other substances i.e. oxidise them