Enzymes
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
What are enzymes made up of
Proteins
What are some organelles that contain catalase
Peroxisomes
Anabolic reactions…
Build things up
Catabolic reactions…
Break things down
What are enzymes
Biological catalysts
Examples of intracellular enzymes
Catalase
Examples of extra cellular enzymes + things that use them
Digestive enzymes- amylase, protease, bacteria, mould
Why is the tertiary structure of enzymes important
Give them their properties
What happens to enzymes in reactions
They are not used up
What can effect enzymes
Temperature and pH
How do enzyme work
By reducing the activation energy
Activation energy
Minimum energy required for a reaction to occur
Types of reaction you can have
Endergonic + exergonic
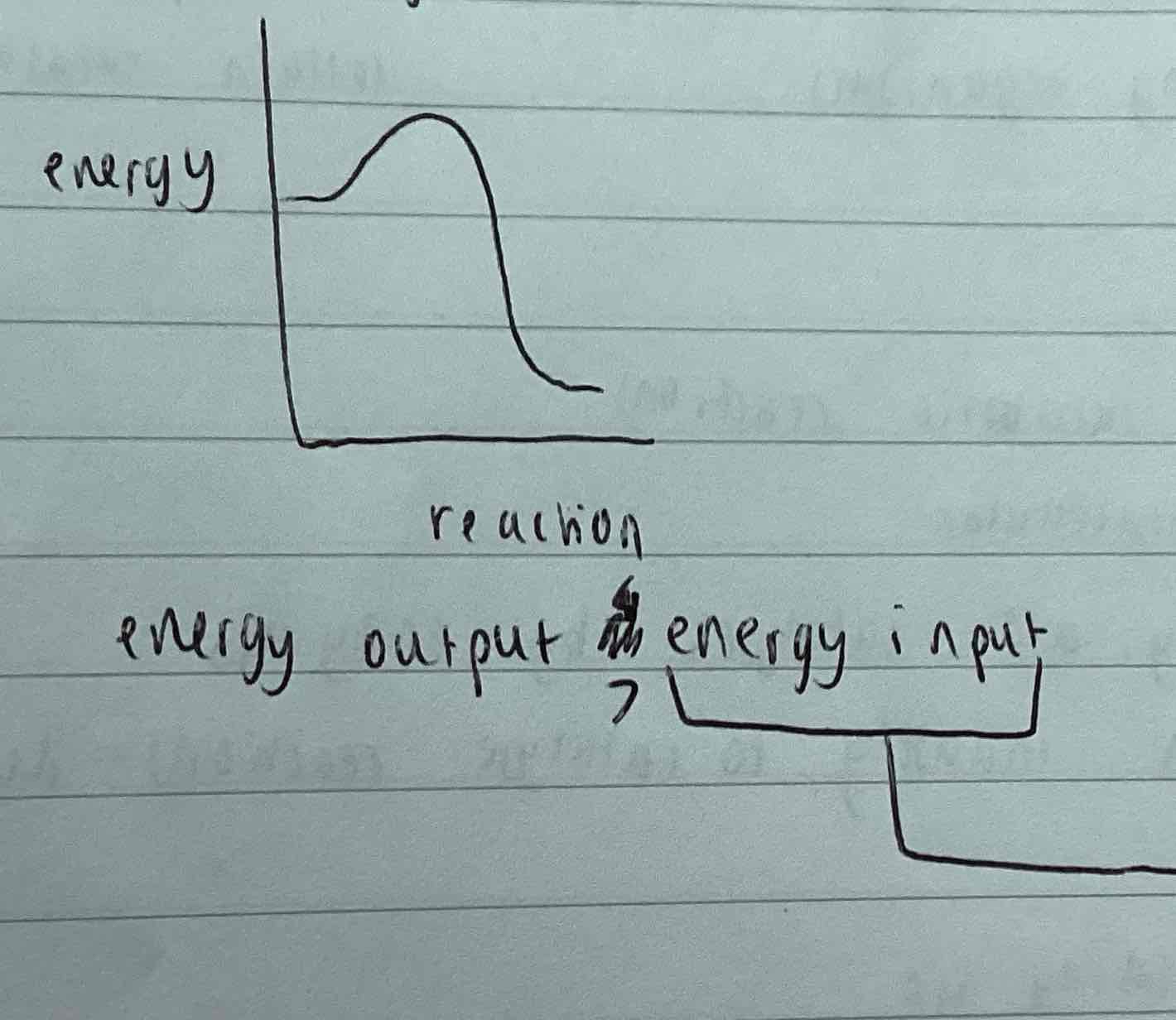
Exergonic reaction
Energy output > energy input / activation energy
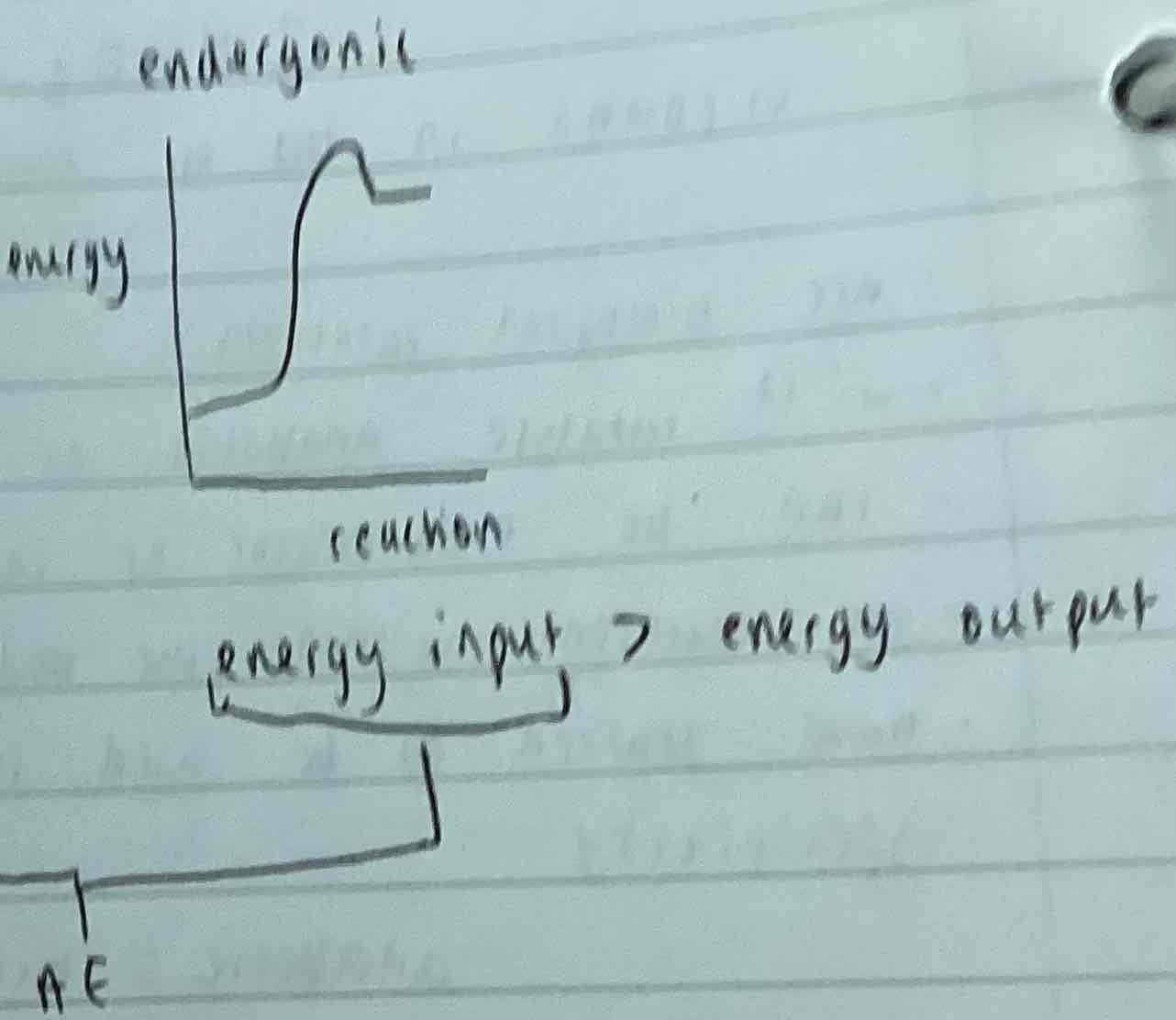
Endergonic reactions
Energy input/ activation energy > energy output
How is activation energy lowered
Reactants are held close together in exposed positions on active sit, there are strains or tensions within the substrate molecule to change their shape to make them fit better, special conditions at active sit eg r groups, prosthetic groups
key inorganic ions
Ca2+, Na+, K+, NH4+, NO3-, HCO3, Cl-, PO4 3-, OH-
Rate of reaction=
1/ time taken
Optimum temperature
The temperature at which the enzyme has the highest rate of reaction
What happens as temperature increases
To the optimum, rate increases as the kinetic energy of enzyme and substrate increases d there are more successful collisions
What may prevent successful collisions
Active sit may to be available- may already be a substrate there
What happens beyond optimum temperature
Enzymes denature as they gain more kinetic energy so the bonds in the tertiary structure vibrate more and eventually break meaning the shape of the active site has changed and is no longer complementary to the substrate
Temperature coefficient
Qn= rate at (t + n degrees)/ rate at t degrees
Lock and key model
Substrate and active site are complementary
Induced fit model
Enzyme and substrate may not be completely complementary so during reaction when r groups in enzymes react with the substrate the shape changes slightly so the substrate fits more snugly
How does pH effect rate
Unlike temp either side of the optimum denaturation occurs as H+ ions can affect the bonding between r groups and may cause ionisation meaning the tertiary structure is changed
Why is the rate fastest at the start
When the reaction first begins there are a lot of successful collisions as the number of free active sites is high and the number of substrates is high
Why does rate decrease with time
Number of successful collisions decreases as substrate molecules are used up and number of active sites decreases
Effect of concentration on rate
Starts at a high rate as chance of successful collisions is greatest
Cofactors
Small inorganic parts of enzymes that they need to work
Common features of cofactors
Are always non protein, often need to be in the active site, often ions, within structure of enzymes
Examples of cofactors
Cl- ions are cofactors for amylase, Fe+ ions are cofactors in catalase
Coenzymes
Organic parts that enzymes need to work
Features of coenzymes
Non protein, large, often have a temporary role, large
Examples of coenzymes
NAD derived from Vito B3 needed in aerobic respiration, coenzymeA derives from vitamin B
What are cofactors+ coenzymes called if they are permanently bonded
They rae prosthetic groups
Competitive inhibitors
Prevent escs from forming as are also complementary
Features of competitive inhibitors
Often reversible, can be naturally occurring, stops reaction, prevent ESCs from forming
Non competitive inhibitors
Is allosteric inhibition that changes the shape of the enzyme by changing its tertiary structure
How do non competitive inhibitors work
Bind to the anyone’s allosteric site rather than its active site
Features of non competitive inhibitors
Changes shape of active site, often irreversible, tend to be drugs or poisons
Compared to the normal rate of a reaction, what happens with a competitive inhibitor
There is a lower rate of reaction- the reaction still goes to completion and max rate of reaction is reached just slower
Compared to the normal rate of reaction what happens when a non competitive inhibitor is introduced
Rate of reaction is slower- max rate/rate without inhibitor is not etched as the number of ESCs are reduced due to the permanent changes that occur
End product inhibition
When the inhibitor is non competitive and the last product
What happens during end product inhibition
The end product binds to an enzyme allosterically earlier in the sequence, inhibiting itself
How does end product inhibition work
Greater inhibition leads to a decrease in amount of end product, inhibition decreases in he conc. of end product decreases- is a negative feedback loop
Intracellular enzymes
Enzymes that act within cells
Extracellular enzymes
Enzymes that are released from the cells to work outside of the cells
Why are extracellular enzymes needed
Large molecules such as nutrients in the forms of polymers such as proteins and polysaccharides are too large to enter the cells directly so need to broken down
Role of extracellular enzymes in single and multi celled organisms
Rely on EE for nutrition - single celled organisms (bacteria and yeast) release EE to break down things such as proteins into amino acids and glucose, multicellular organism use them to break things down in the digestive system
How is starch digested
Digested in two steps with two different enzymes
First step of starch digestion
Starch polymers are partly broken down into maltose using the enzyme amylase
Where is amylase produced
Saliva, pancreatic juices in small intestine
Stage 2 of starch digestion
Maltose is broken down into glucose using enzyme maltase
Where is maltase found
Small intestine
Which enzyme catalyses the digestion of proteins
Protease enzymes
How are proteins digested
Broken down by protease enzymes into smaller peptides that can be broken down in to amino acids which are then absorbed by cells lining the digestive system to be absorbed by bloodstream
Example of a protease enzyme
Trypsin
Where is trypsin produced and where is released to
Produced in pancreas, released into smn all intestine with the pancreatic juices
How to calculate rate of reaction for a gas producing reaction
Volume/time