Bio 150 Exam 1 - Ch. 1,2,3
4.0(1)
4.0(1)
Card Sorting
1/31
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
1
New cards
Briefly describe the unifying themes that characterize the biological sciences.
Order, regulation, energy processing, growth & development, reproduction, responsiveness, evolutionary adaption
2
New cards
Identify the three domains of life and the three kingdoms of multicellular, eukaryotic life.
Domain Archaea - Prokaryotic (unicellular, simple cells)
Domain Bacteria - Prokaryotic (unicellular, simple cells)
Domain Eukarya - Eukaryotic (complex, diff types of organelles)
* Kingdom Animalia → Animals obtain food by ingesting other organisms
* Kingdom Fungi → Fungi are decomposers that absorb nutrients by breaking down dead organisms and organic wastes.
* Kingdom Plantae → Plants produce their own sugars and food by photosynthesis.
Domain Bacteria - Prokaryotic (unicellular, simple cells)
Domain Eukarya - Eukaryotic (complex, diff types of organelles)
* Kingdom Animalia → Animals obtain food by ingesting other organisms
* Kingdom Fungi → Fungi are decomposers that absorb nutrients by breaking down dead organisms and organic wastes.
* Kingdom Plantae → Plants produce their own sugars and food by photosynthesis.
3
New cards
Explain how evolution accounts for the unity and diversity of living things.
Evolution and diversity result from the interactions between organisms and their environments and the consequences of these interactions over long periods of time.
4
New cards
Describe the observations and inferences that led Charles Darwin to his theory of evolution by natural selection.
1. Individuals in a population vary in their traits (many seem heritable)
2. A population can produce far more offspring than can survive to produce offspring of their own (competition is inevitable)
3. Species generally are suited to their environments (adapted to their circumstances)
Inferences: Individuals with inherited traits that are better suited to the local environment are more likely to survive and reproduce; over time a higher proportion of the population will have the advantageous traits
5
New cards
Natural Selection
Unequal reproductive success of individuals ultimately leads to adaption to their environment as long as the environment remains the same
6
New cards
Describe the steps of the scientific method.
1. Question
2. Research
3. Hypothesis
4. Experiment
5. Observation/Data
6. Conclusion
7
New cards
Distinguish between quantitative and qualitative data.
Quantitative: data is collected through trials that produce numerical data, such as measurements.
Qualitative: data collected by using the senses(hear, smell) from what a person observes.
Qualitative: data collected by using the senses(hear, smell) from what a person observes.
8
New cards
Explain why hypotheses must be testable and falsifiable but are not provable
A hypothesis is a tentative explanation that answers a question from observation. It can be shown to be false, but never true.
9
New cards
Describe what is meant by a controlled experiment.
An experiment designed to compare an experimental group with a control group
10
New cards
Distinguish between the following pairs of terms:
a. control and experimental groups
b. dependent and independent variables
c. positive and negative controls
a. control and experimental groups
b. dependent and independent variables
c. positive and negative controls
a. An experimental group receives the treatment whose effect researchers wish to study, whereas a control group does not
\
b. Independent: the factor being manipulated
Dependent: the factor being measured that is predicted to be affected by the independent variable
\
c. A positive control group is a group in the experiment that is given a treatment with a known outcome, while a negative control group is given no special treatment at all.
\
b. Independent: the factor being manipulated
Dependent: the factor being measured that is predicted to be affected by the independent variable
\
c. A positive control group is a group in the experiment that is given a treatment with a known outcome, while a negative control group is given no special treatment at all.
11
New cards
Describe the parts of a graph and relate variables to a graph.
T - Title
A - Axis (independent on x-axis; dependent on y)
I - Interval
L - Label
S - Scale
A - Axis (independent on x-axis; dependent on y)
I - Interval
L - Label
S - Scale
12
New cards
Explain how a scientific theory is different from a hypothesis
A theory is much broader in scope than a hypothesis, a theory is general enough to spin off many new testable hypotheses, and a theory is generally supported by a much greater body of evidence
13
New cards
Distinguish between an element and a compound.
Element: Substance that cannot be broken down into other substances by chemical reactions
Compound: A substance consisting of two or more different elements combined in a fixed ratio
Compound: A substance consisting of two or more different elements combined in a fixed ratio
14
New cards
Identify the major elements that make up living matter. (CHNOPS)
Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur
15
New cards
Define the term trace element and give examples
Trace elements are required by an organism in only minute species (ex. iron or zinc)
16
New cards
Draw and label a simplified model of an atom
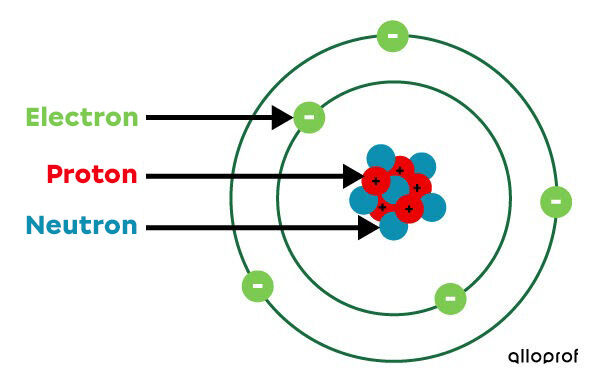
17
New cards
Distinguish between each of the following pairs of terms:
a. neutron and proton
b. atomic number and mass number
c. atomic weight and mass number
a. neutron and proton
b. atomic number and mass number
c. atomic weight and mass number
a. neutron is electrically neutral; proton is positively charged
b. atomic number is the # of protons (bottom); mass number is protons + neutrons (top)
c. atomic mass is the average weight of an element form; mass # is the weight of the nucleus of an atom
b. atomic number is the # of protons (bottom); mass number is protons + neutrons (top)
c. atomic mass is the average weight of an element form; mass # is the weight of the nucleus of an atom
18
New cards
Explain how the atomic number and mass number of an atom can be used to determine the number of neutrons
mass number - atomic number = # of neutrons
19
New cards
Explain how two isotopes of an element are similar and different, and describe biological applications of radioactive isotopes
Have the same number of protons, but different number of neutrons and thus mass (behaves identically in chemical reactions)
1. used in fossils to date those relics of past life.
2. used as tracers to follow atoms through metabolism, the chemical process of an organism.
1. used in fossils to date those relics of past life.
2. used as tracers to follow atoms through metabolism, the chemical process of an organism.
20
New cards
Define the terms energy and potential energy, and relate potential energy to electron shells
Energy: the capacity to cause change
Potential energy: the energy that matter possesses because of its location or structure
The electrons present in the outer electron shells have more potential energy with reference to the nucleus.
Potential energy: the energy that matter possesses because of its location or structure
The electrons present in the outer electron shells have more potential energy with reference to the nucleus.
21
New cards
Distinguish between nonpolar covalent, polar covalent, ionic and hydrogen bonds
Nonpolar covalent: electrons shared equally between 2 atoms
Polar covalent: electrons shared unequally
ionic bonds: the attraction between cations and anions
hydrogen bonds: the attraction between a hydrogen and an electronegative atom
Polar covalent: electrons shared unequally
ionic bonds: the attraction between cations and anions
hydrogen bonds: the attraction between a hydrogen and an electronegative atom
22
New cards
Explain why strong covalent bonds and weak bonds are both essential in living organisms
Strong covalent bonds link atoms to form cell molecules like hydrogen, oxygen, and nitrogen atoms.
Weak bonds contribute greatly to the development properties of life.
Weak bonds contribute greatly to the development properties of life.
23
New cards
With the use of a diagram, explain why water molecules are:
a. polar
b. capable of hydrogen bonding with 4 neighboring water molecules
a. polar
b. capable of hydrogen bonding with 4 neighboring water molecules
a. The water molecule is polar because oxygen has a slight negative charge and hydrogen has a slight positive charge.
b. It is capable of bonding with four neighboring water molecules because each molecule can hydrogen bond to a maximum of four other water molecules. Four emergent properties are cohesion, adhesion, surface tension, and high specific heat
b. It is capable of bonding with four neighboring water molecules because each molecule can hydrogen bond to a maximum of four other water molecules. Four emergent properties are cohesion, adhesion, surface tension, and high specific heat
24
New cards
Explain four characteristics of water that result from hydrogen bonding.
1. Surface Tension: measure of how difficult it is to stretch/break the surface of a liquid.
2. Cohesion: Water molecules attracted to other water molecules.
3. Adhesion: Water molecules attracted to other objects.
4. Capillary Action: Teamwork of cohesion and adhesion draws water up.
25
New cards
Define cohesion and adhesion. Explain how water’s cohesion and adhesion contribute to the movement of water from the roots to the leaves of a tree
Cohesion: Water molecules attracted to other water molecules.
Adhesion: Water molecules attracted to other objects.
Adhesion makes the water stick to the tree and cohesion makes it travel up the roots.
Adhesion: Water molecules attracted to other objects.
Adhesion makes the water stick to the tree and cohesion makes it travel up the roots.
26
New cards
Explain the following observations by referring to the properties of water:
a. Coastal areas have milder climates than adjacent inland areas.
b. Ocean temperatures fluctuate much less than temperatures on land.
c. Insects like water striders can walk on the surface of a pond without breaking the surface.
d. If you slightly overfull a water glass, the water will form a convex surface above the top of the glass
e. If you place a paper towel so that it touches spilled water, the towel will draw in the water
f. Ice floats on water
g. Humans sweat and dogs pant to cool themselves on hot days
a. Coastal areas have milder climates than adjacent inland areas.
b. Ocean temperatures fluctuate much less than temperatures on land.
c. Insects like water striders can walk on the surface of a pond without breaking the surface.
d. If you slightly overfull a water glass, the water will form a convex surface above the top of the glass
e. If you place a paper towel so that it touches spilled water, the towel will draw in the water
f. Ice floats on water
g. Humans sweat and dogs pant to cool themselves on hot days
a) Water is able to absorb large amounts of energy, including heat. So the ocean absorbs heat in the summer because the water is cooler than the air, sucking the heat out of the air, thus cooling the air in summer. In the winter, the air is cooler than water, so the heat moves from the water to the air, warming the air in winter.
\n b) Water is able to absorb large amounts of energy, including heat, and is denser as a liquid than a solid. Therefore, it takes a lot of energy to heat water, and water does not give up it's heat as quickly as air does -- high specific heat and high heat of vaporization
\n c) (surface tension) The cohesive property of water -- the tendency of water to be attracted to water, makes the top layer of water molecules that don't have any water above them are pulled inward to other molecules, so when a water strider steps on water, the force of the top layer of water coheres to itself and resists the foot of the water strider from separating water molecules from each other.
\
d) The water is being absorbed, or soaked up, by the paper towel material through a process called capillary action (the rising or absorption of liquids through small gaps)
\n e) The cohesive property of water -- the water at the top of the overfilled glass is more attracted to the the water than to the side of the glass, creating a convex meniscus
\n f) Water expands as it changes from a liquid to a solid, becoming less dense rather than more dense
\n g) High specific heat Water absorbs latent heat in order to evaporate, and this absorption of heat causes a cooling for sweating humans and panting dogs.
\n b) Water is able to absorb large amounts of energy, including heat, and is denser as a liquid than a solid. Therefore, it takes a lot of energy to heat water, and water does not give up it's heat as quickly as air does -- high specific heat and high heat of vaporization
\n c) (surface tension) The cohesive property of water -- the tendency of water to be attracted to water, makes the top layer of water molecules that don't have any water above them are pulled inward to other molecules, so when a water strider steps on water, the force of the top layer of water coheres to itself and resists the foot of the water strider from separating water molecules from each other.
\
d) The water is being absorbed, or soaked up, by the paper towel material through a process called capillary action (the rising or absorption of liquids through small gaps)
\n e) The cohesive property of water -- the water at the top of the overfilled glass is more attracted to the the water than to the side of the glass, creating a convex meniscus
\n f) Water expands as it changes from a liquid to a solid, becoming less dense rather than more dense
\n g) High specific heat Water absorbs latent heat in order to evaporate, and this absorption of heat causes a cooling for sweating humans and panting dogs.
27
New cards
Distinguish between a solute, a solvent and a solution
Solute: dissolved substance
Solvent: dissolving agent of a solution
Solution: A liquid that is a completely homogeneous mixture of two or more substances
Solvent: dissolving agent of a solution
Solution: A liquid that is a completely homogeneous mixture of two or more substances
28
New cards
Distinguish between hydrophobic and hydrophilic substances
Hydrophobic: Repels water bc they are nonionic and nonpolar
Hydrophilic: Any substance that has an affinity for water
Hydrophilic: Any substance that has an affinity for water
29
New cards
Name the products of the dissociation of water and give their concentration in pure water
Hydronium ions (H3O+) and hydroxide ions (OH-) are the products.
The concentration of these ions at 25 C is 10^-7 M each.
The concentration of these ions at 25 C is 10^-7 M each.
30
New cards
Define acid, base, and pH
Acid: a substance that increases the hydrogen ion concentration of a solution
Base: a substance that reduces the hydrogen ion concentration of a solution
pH: the negative logarithm of the hydrogen ion concentration
Base: a substance that reduces the hydrogen ion concentration of a solution
pH: the negative logarithm of the hydrogen ion concentration
31
New cards
Explain how acids and bases may directly or indirectly alter the hydrogen ion concentration of a solution
Acids increase the hydrogen ion concentration in a substance and bases decrease it.
32
New cards
Explain how buffers work
Buffer: a substance that minimizes changes in the concentrations of H+ and OH- in a solution. It does so by accepting hydrogen ions from the solution when they are in excess and donating hydrogen ions to the solution when they have been depleted.