4.1.1.2 Mixtures
0.0(0)
0.0(0)
Card Sorting
1/15
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
1
New cards
What is a mixture
Two or more substances not chemically combined together
2
New cards
Soluble
Solid substances that **can** dissolve in liquids are described as Soluble
3
New cards
Insoluble
Solids that **cannot** dissolve are described as insoluble
4
New cards
Solute
5
New cards
Solvent
A solvent is a liquid in which substances can dissolve.
6
New cards
Solution
soluble Solute (solid) and solvent
7
New cards
What does filtration do
Separate insoluble solids from liquids
8
New cards
Method of filtration
1. One beaker contains a mixture of solid and liquid, the other contains a funnel with filter paper.
2. The solid and liquid mixture is poured into the filter funnel
3. The liquid drips through the filter paper but the solid particles are caught in the filter paper.
4. the sand stays behind in the filter paper, it becomes the residue. the water passes through the filter paper, it becomes the filtrate.
\
9
New cards
Why is filter paper used
Filter paper is full of small holes that are large enough to allow liquid particles to move through, but too small to allow solids through. This separates the solids from the liquids in filtration.
The filter paper has lots of tiny holes in it, the sand stays behind in the filter paper, it becomes the **residue**.
the water passes through the filter paper, it becomes the **filtrate**. are far too small to see.
The **particles** of the liquid including any dissolved **solutes**will pass through the holes in the filter paper.
If the liquid contains an **insoluble** solid made of large particles, these larger particles cannot pass through the holes in the filter paper.
The filter paper has lots of tiny holes in it, the sand stays behind in the filter paper, it becomes the **residue**.
the water passes through the filter paper, it becomes the **filtrate**. are far too small to see.
The **particles** of the liquid including any dissolved **solutes**will pass through the holes in the filter paper.
If the liquid contains an **insoluble** solid made of large particles, these larger particles cannot pass through the holes in the filter paper.
10
New cards
What is crystallisation/ evaporation used for
Soluble solid in a liquid which has dissolved (solutions)
11
New cards
crystillation method
First, pour the solution into an evaporating dish and heat it using a Bunsen burner.
Slowly heat solution- solvent will evaporate and the solution will get more concentrated
Stop heating it when crystals start to form ('point of crystallisation') and allow it to cool down.
Then either leave it to allow the rest of the water to evaporate, or filter out the crystals using filter paper and a funnel.
Lastly, dry the crystals in a drying oven or desiccator
Slowly heat solution- solvent will evaporate and the solution will get more concentrated
Stop heating it when crystals start to form ('point of crystallisation') and allow it to cool down.
Then either leave it to allow the rest of the water to evaporate, or filter out the crystals using filter paper and a funnel.
Lastly, dry the crystals in a drying oven or desiccator
12
New cards
Seperating a rock salt
* Rook salt is simply a mixture of salt and sand
* Salt and sand are both compounds - but salt dissolves in water and sand doesn't. This vital difference in their physical properties gives a great way to separate them.
1. Grind the mixture to make sure the salt crystals are small, so will dissolve easily.
2. Put the mixture in water and stir. The salt will dissolve, but the sand won't. Heat mixture help dissolve the salt.
3. Filter the mixture. The grains of sand won't fit through the tiny holes in the filter paper, so they collect on the paper instead. The salt passes through the filter paper as it's part of the solution.
4. Evaporate the water from the salt so that it forms dry crystals.
* Salt and sand are both compounds - but salt dissolves in water and sand doesn't. This vital difference in their physical properties gives a great way to separate them.
1. Grind the mixture to make sure the salt crystals are small, so will dissolve easily.
2. Put the mixture in water and stir. The salt will dissolve, but the sand won't. Heat mixture help dissolve the salt.
3. Filter the mixture. The grains of sand won't fit through the tiny holes in the filter paper, so they collect on the paper instead. The salt passes through the filter paper as it's part of the solution.
4. Evaporate the water from the salt so that it forms dry crystals.
13
New cards
Chromatography method
**This technique can be used to separate different dues in an ink.**
1. Draw a line near the bottom of a sheet of filter paper.
2. Add a spot of the ink to the line and place the sheet in a beaker of solvent, e.g water.
3. The solvent used depends on what's being tested. Some compounds dissolve well in water, but sometimes other solvents, like ethanol, are needed.
4. Make sure the ink isn't touching the solvent- you don't want it to dissolve into it.
5. Place a lid on top of the container to stop the solvent evaporating
6. The solvent seeps up the paper, carrying the ink with it.
7. Each different due in the ink will move up the paper at a different rate so the dyes will separate out. Each dye will form a spot in a different place - 1 spot per dye in the ink.
8. If any of the dyes in the ink are insoluble (won't dissolve) in the solvent you've used, they'll stay on the baseline.
9. When the solvent has nearly reached the top of the paper, take the paper out of The point the solvent has the beaker and leave it to dry.
reached as it moves up the
10. The end result is a pattern of spots called a chromatogram.
1. Draw a line near the bottom of a sheet of filter paper.
2. Add a spot of the ink to the line and place the sheet in a beaker of solvent, e.g water.
3. The solvent used depends on what's being tested. Some compounds dissolve well in water, but sometimes other solvents, like ethanol, are needed.
4. Make sure the ink isn't touching the solvent- you don't want it to dissolve into it.
5. Place a lid on top of the container to stop the solvent evaporating
6. The solvent seeps up the paper, carrying the ink with it.
7. Each different due in the ink will move up the paper at a different rate so the dyes will separate out. Each dye will form a spot in a different place - 1 spot per dye in the ink.
8. If any of the dyes in the ink are insoluble (won't dissolve) in the solvent you've used, they'll stay on the baseline.
9. When the solvent has nearly reached the top of the paper, take the paper out of The point the solvent has the beaker and leave it to dry.
reached as it moves up the
10. The end result is a pattern of spots called a chromatogram.
14
New cards
Why is the line pencil not pen
Use a pencil to do this - pencil marks are insoluble and won't dissolve in the solvent.
\
\
15
New cards
Simple distillation method
1. Simple distillation is used for separating out a liquid from a solution.
2. The solution is heated. The part of the solution that has the lowest boiling point evaporates first.
3. The vapour is then cooled, condenses (turns The back into a liquid) and is collected.
4. The rest of the solution is left behind in the flask.
5. You can use simple distillation to get pure water from seawater. The water evaporates and is condensed and collected. Eventually you'll end up with just the salt left in the flask.
6. The problem with simple distillation is that you can only use it to separate things with very different boiling points - if the temperature goes higher than the boiling point of the substance with the higher boiling point, they will mix again.
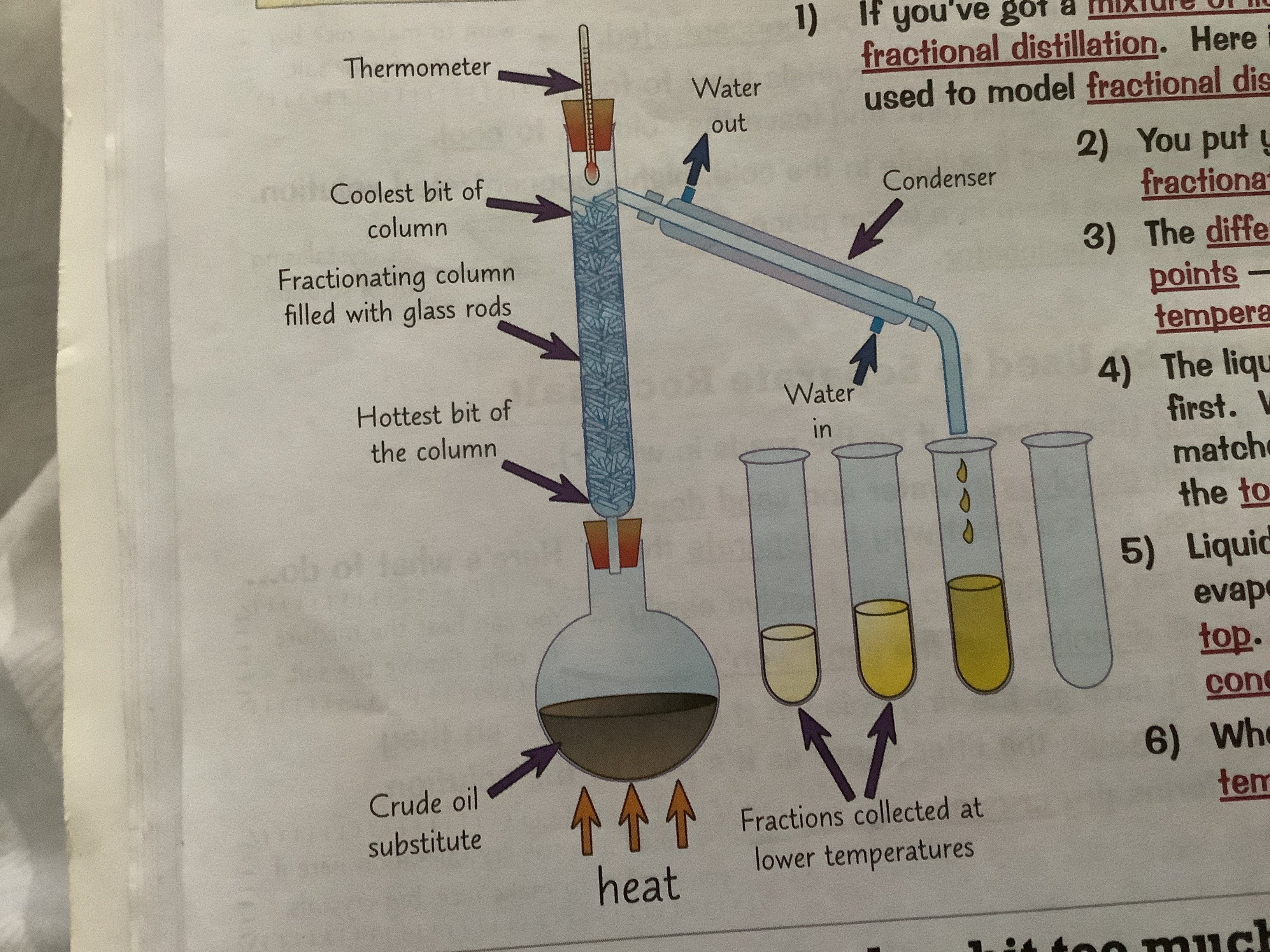
16
New cards
Fractional distillation method
1. If you have a mixture of liquids with similar boiling points you need Pure another method to separate them fractional distillation
2. If you got a mixture of liquids, you can separate it using fractional distillation. Here is a lab demonstration that can be used to model fractional distillation of crude oil at a refinery
3. You put your mixture in a flask and stick a fractionating column on top. Then you heat it
4. The different liquide will all have different bolting points - so they will evaporate at different temperatures.
5. The liquid with the lowest boiling point evaporates first. When the temperature on the thermometer matches the boiling point of this liquid, it will reach the top of the column.
6. Liquids with higher boiling points might also start to evaporate. But the column is cooler towards the top. So they will only get part of the way up before condensing and running back down towards the fed
7. When the first liquid has been collected, you raise temperature until the next one reaches the top.