Thermal Energy Transfers: Kinetic Theory, Temperature, and System Properties
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Internal Energy
The energy contained in substances by the particles/molecules they are made of having kinetic and potential energy.
Thermal Energy Transfer
Energy is always transferred from hotter (higher temperature) objects to cooler (lower temperature) objects.
Kinetic Theory of Matter
The idea that all matter is made of countless billions of particles constantly moving in various ways with forces acting between them when they get close enough.
Kinetic Molecular Theory (KMT)
Another term used to refer to the kinetic theory of matter.
Density
A useful measure of substances based on the ratio of its mass and volume.
Temperature
A way to measure the average kinetic energy of particles of a substance.
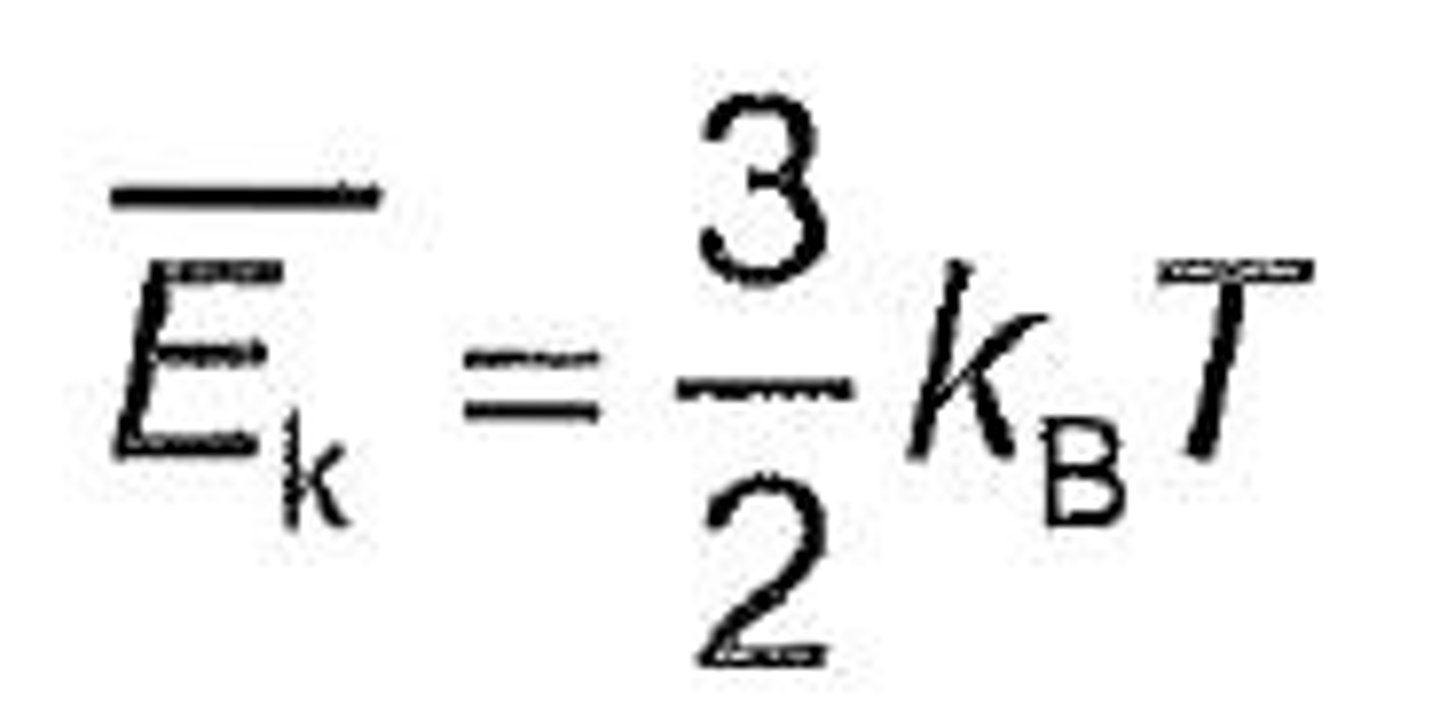
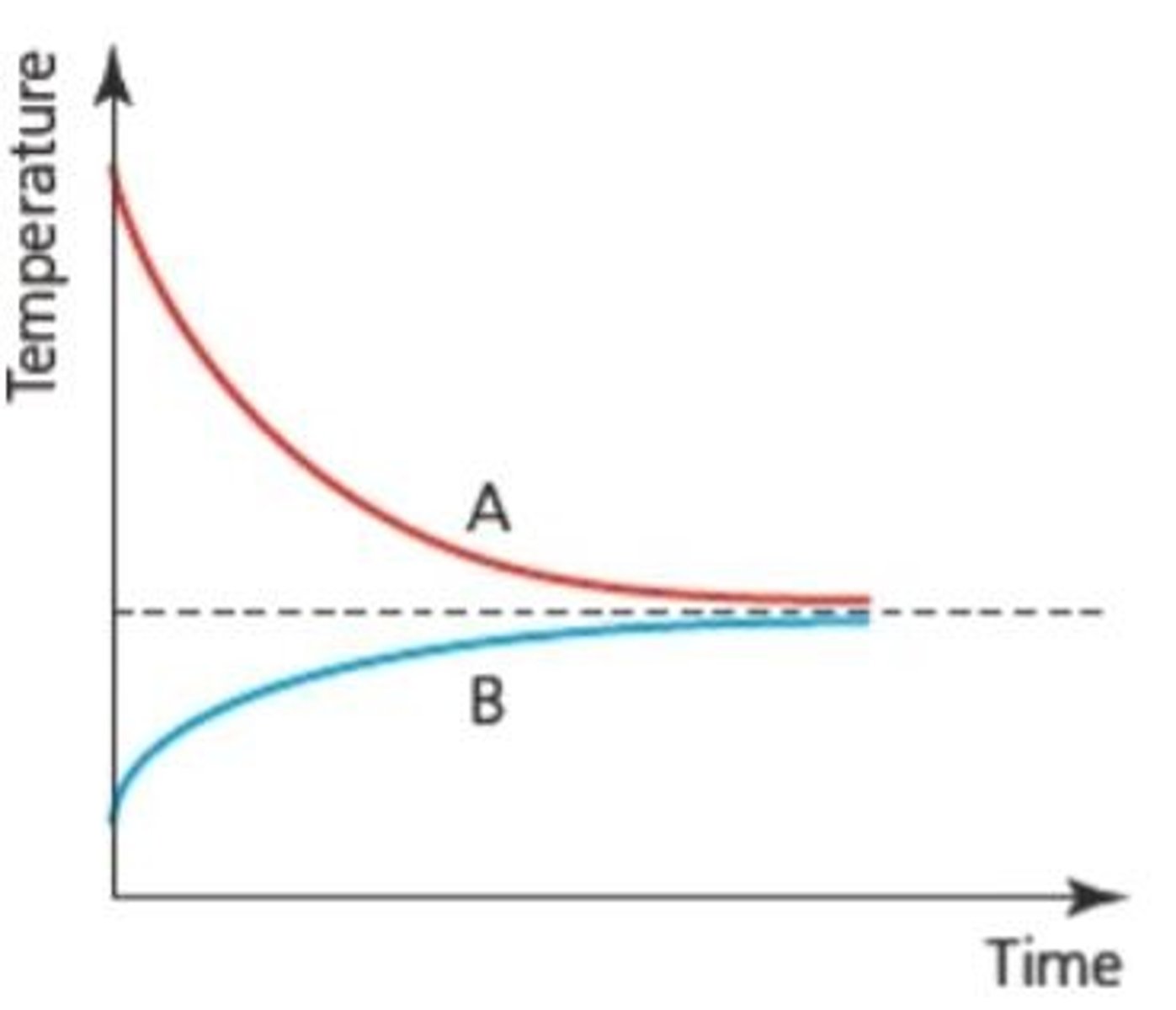
Thermal Equilibrium
If objects are at the same temperature there will be no net energy transfer between them.
Celsius Temperature Scale
Based on the boiling and freezing points of water under specific conditions.
Kelvin Temperature Scale
Considered an absolute scale as zero is absolute zero, the temperature where molecular motion essentially stops.
Absolute Zero
The temperature where molecular motion essentially stops, equivalent to -273.15 °C on the Celsius scale.
Temperature Difference
Determines the direction of the resultant thermal energy transfer between bodies.
Change in Temperature
The same when expressed in Kelvin or Celsius scales.
Flow of Thermal Energy
Will always be from higher temperature to lower temperature.
Increase in Temperature
Equivalent to the particles gaining kinetic energy.
Crystalline Structure of Ice
When water changes from liquid to solid, it forms a crystalline structure in which the volume increases.
Ice Floats in Water
A phenomenon due to the crystalline structure of ice, allowing many forms of life to exist on earth.
One Degree of Celsius and Kelvin
One degree of Celsius and one degree of Kelvin are the same.
Macroscopic Observations
Provide a model of the microscopic properties of a substance.
Energy Transfer within Systems
Describes how energy is transferred within and between systems.
Observations of Physical Quantities
Can be used to determine the other properties of a system.