Lesson 2 - The electron configuration
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Dmitri Ivanovich Mendeleev
developer of periodic table
Groups
_______ of the periodic table are displayed as vertical columns numbered from 1 to 18. Each carries their own distinct chemical properties and can be classified into four families
representative, transition, inner transition, and noble gases
Period
displayed as horizontal rows numbered from 1 to 7
Blocks
_____ of the periodic table are the last subshell of the elements and are classified into s, p, d, f.
Atoms
smallest unit. something that is uncuttable or which cannot be split. All the elements are made from these.
Atomos, Greek
origin of the word atom
The electron shells
according to the Bohr model, the electrons occupy fixed circular orbits around the nucleus of an atom. Each electron has a different energy level and the closest one to the nucleus have the lowest energy.
Valence Electrons
Electrons in the outermost shells of atoms are called _____.
2
6
10
14
Give the numbers of electrons that these can give (e.g. 1,9,8,7)
s subshell -
p subshell -
d subshell -
f subshell -
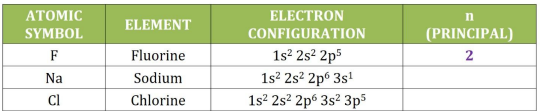
Principal (n)
is the distance of electrons from the nucleus and is identified as the outermost occupied shell given in the configuration
example:
1s^2 2s^2 2p^5
n = 2
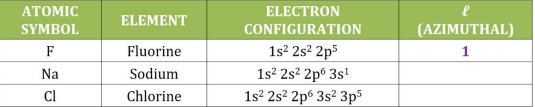
Azimuthal (l)
the shape of the orbitals and is identified as the sublevel where an electron is located in the configuration.
example: 1s² 2s² 2p^5
l = p, p=1
0
1
2
3
Give all the azimuthal values of the following
s -
p -
d -
f -
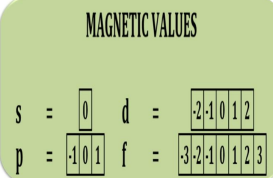
Magnetic
orientation in the space of the electron configuration and has values ranging from positive to negative
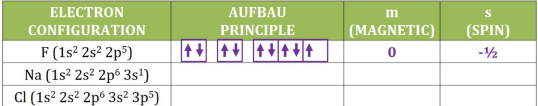
Spin
the spin is the possible direction in which an electron spin occurs
½ or -1/2