Chemistry - Atomic Structure and the Periodic Table
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
What are the initial postulates of Dalton’s Theory? (4)
All atoms of the same element are exactly alike
Atoms cannot be broken down any further
Atoms of different elements have different masses
Atoms combine to form complex structures
State relevant modifications to Dalton’s Theory that support modern evidence (8)
Atoms of the same element are not all exactly alike due to the existence of isotopes
In 1886, Eugene Goldstein discovered protons
In 1897, Sir Joseph John Thomson discovered the electrons and suggested the plum-pudding model
In 1909, Ernest Rutherford suggested the planetary model
In 1913, Niels Bohr suggested that electrons could only orbit the nucleus at certain distances depending on their energy
In 1926, Erwin Schrödinger, Werner Heisenberg and Max Born defined the probability of finding an electron at any one point, leading to the development of atomic orbitals
In 1932, James Chadwick discovered neutrons
Describe the structure of an atom and its subatomic particles (4)
The atom is composed of its subatomic particles, the nucleus and its energy levels
Electrons are found in the energy levels, have a relative mass of 1/1840 and a relative charge of -1
Neutrons are found in the nucleus, have a relative mass of 1 and a relative charge of 0
Protons are found in the nucleus, have a relative mass of 1 and a relative charge of +1
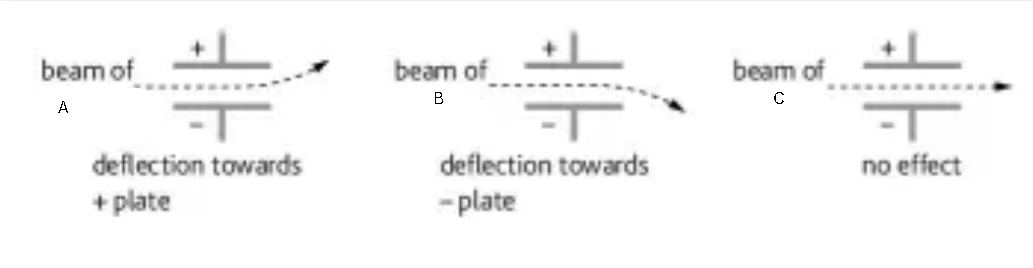
What is this diagram? Label it
Subatomic particles in electric field
A - electrons
B - protons
C - neutrons
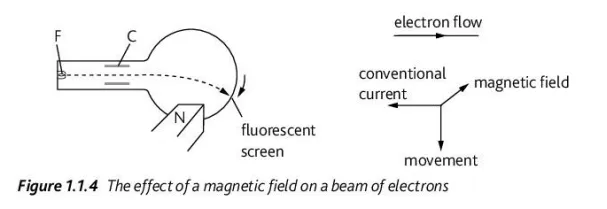
Describe the movement of subatomic particles in a magnetic field and the apparatus required to observe this (3/4)
A strong magnetic field causes electrons to move in a downwards perpendicular direction from a convectional current and magnetic field, while it causes protons to move in the opposite direction of that of the electrons. The neutrons will not be deflected
In the apparatus, the electrons are produced from a tungsten filament, F, from a low voltage supply and are accelerated towards the metal cylinder, C. Eventually, they collide with the fluorescent screen, producing a glow and the glow eventually descends when the north pole of a magnet (N, positively charged) is brought near it
isotope
atoms with the same atomic numbers but different mass numbers than a typical atom from that element
Differentiate between atomic number, mass number, relative atomic mass and relative isotopic mass
Atomic number refers to the number of protons an atom has
Mass number refers to the number of protons and neutrons an atom has
Relative atomic mass describes the weight of a naturally occurring atom that is proportionate to the mass of a carbon-12 atom with the consideration of the proportion of isotopes in the naturally occurring element
Relative isotopic mass describe the weight of an isotope that is proportionate to the mass of carbon-12 atom
radioactive isotope
an isotope whose nucleus randomly breaks down spontaneously in order to achieve stability
emissions
rays of particles that are given out when the nuclei of isotopes decay
What are the types of emissions? (3)
Alpha, beta and gamma
Describe alpha emissions
the instance in which a nucleus ejects a particle equivalent to a helium-4 nucleus
The isotope produced by these emissions have a mass number 4 units lower and a nuclear 2 units low than the original atom
They can be stopped by a thin sheet of paper
Describe beta emissions
the emission of a neutron that transforms into a proton and an electron
The isotopes produced by beta emissions have the same mass number but the number of protons increases by one
These can be stopped by 6mm thick aluminum foil
Describe gamma emissions and electron capture
instance in which a nucleus decays and emits high frequency electromagnetic radiations
These can be stopped by a thick lead sheet
Electron capture describes the instance in which gamma rays are emitted alongside beta or alpha particles in which a proton is converted into a neutron, where the mass number stays the same but the atomic number decreases by one
Describe the use of radioactive isotopes in tracers, cancer treatment, organism dating, smoke detectors and power generation (5)
Iodine-131 is used to study thyroid function as a tracer
Radiotherapy is used to treat cancer
Carbon-14 is used to date organisms
Americium-241 is used in smoke detectors
Uranium-235 is used in power generation in nuclear reactors
Describe an energy level
fixed distances from the nucleus where an electron may be found
Describe emission spectrum and how it proves the existence of discrete energy lines
An emission spectrum is a display of the electromagnetic radiation emitted by the atoms of elements
These emissions arise when electrons are transitioning between different energy levels. In order for an electron to occupy a particular energy level it has to have the exact amount of energy present at said level.
The farther from the nucleus, the more energy present at each energy level. When an electron is transitioning to a higher energy level, it absorbs energy and when it transitions to a lower energy, it emits energy. This energy is in the form of a photon.
In order for an electron to transition, it has to absorb or emit a photon that has an energy equivalent to the energy difference between its current level and the level it wants to go to
When electrons transition to lower elements, they emit photons with this particular wavelengths which correspond with a particular type of light and this is observed on a spectrometer.
Theoretically, if the energy levels were not discrete, a continuous spectrum would be displayed as a result of the range of the varying wavelengths of the photons that would have to be emitted.
Instead, discrete lines are displayed representing the fixed wavelengths of photons being emitted — indicating that the energy difference between energy levels is fixed and by extension that energy levels are discrete

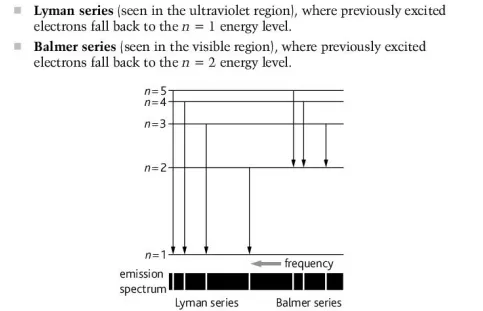
Describe the Lyman and Balmer series
The Lyman series (3 eV to 124eV) refers to the spectral series which describes the energy range that coincides with ultraviolet light
The Balmer series (1.77 eV to 3.1eV) refers to the spectral series which describes the energy range that corresponds with visible light
Describe the hydrogen emission spectrum in relation to the Lyman and Balmer series
When an electron transmissions to the energy level n = 1, it emits a photon that coincides with the Lyman series
When an electron transmissions to the energy level n = 2, it emits a photon that coincides with the Balmer series
sublevel
divisions within energy levels which describe the capacity of each energy level to hold electrons that coincide with varying amounts of atomic orbitals
atomic orbital
a region of space within a sublevel where electrons are likely to be found
Describe the first three sublevels
s sub-level holds1 orbital and 2 electrons
p sub-level holds 2 orbitals and 6 electrons
d sub-level holds 5 orbitals and 10 electrons
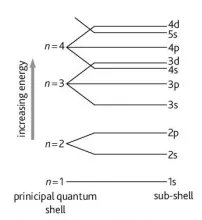
Describe the arrangement of the atomic orbitals in terms of energy levels
Describe the shape of s and p orbitals (4)
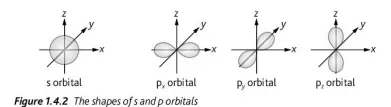
ionization energy
the energy required to remove an electron from an atom
first ionization energy
energy needed to move on electron from each atom in a mole of atoms of an element in its gaseous form to form one mole of gaseous ions
Describe the effect of nuclear charge, distance between nucleus and valence electrons and shielding affect ionization energy
The higher the nuclear charge, the greater the electrostatic forces between the nucleus and electrons — increasing the amount of ionization energy needed to remove said electrons
The further the valence electrons are from the nucleus, the weaker the electrostatic forces are between them and the nucleus — lowering the amount of ΔHᵢ₁ needed to remove said electrons
The more full inner shells between the valence shell and nucleus, the greater the shielding effect — lowering the amount of ionization energy needed to remove said electrons
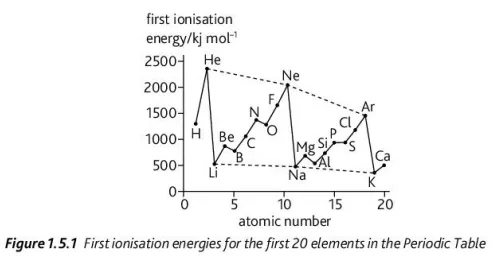
Using this graph, explain the change in ionization energy when a new period starts
There’s a sharp decrease in ionization energy because the valence electron goes into an orbital further from the nucleus, as the previous inner shell would be full — increasing shielding and decreasing the amount of ionization energy needed
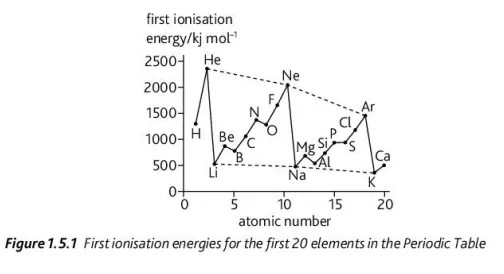
Using this graph, explain the change in ionization energy from Be → B and Mg → Al
This is due to the electrons going into sublevels that are slightly farther from the nucleus
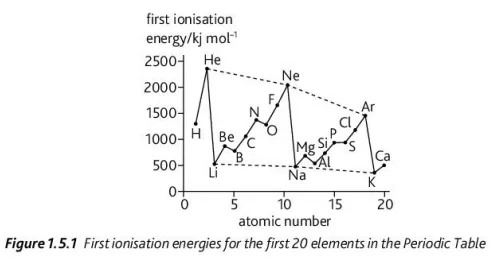
Using this graph, explain the change in ionization energy in N → O and P → S
This can be accredited to same-pair repulsion as electrons naturally repel each other due to their like-charges so when two electrons are in the same orbital the repulsion between them increases and weakens their electrostatic attraction to the nucleus, making it easier for them to be ionized
successive ionization energy
the energy needed to remove an electron after the first electron