chem 51a midterm 2
1/27
Earn XP
Description and Tags
prof vanderwal
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
chair conformation stability
if you have one group on the ring the more stable chair will be the one with the group in an equatorial position
two groups best if they occupy equatorial position
if only one can be equatorial in either chair then the more stable will be the one with the larger group in the equatorial position
wedge and dash
wedged = up
dash = down
*no correlation between up/down and axial/equatorial
newman
most stable is staggered specifically anti
cis
two groups are up or down
trans
one group is up and the other is down
gauche interactions in in cyclohexane rings
2 guache interactions for each axial groups in cyclohexane ring
1 guache interaction for each equatorial in cyclohexane ring
methane
1
ethane
2
propane
3
butane
4
pentane
5
hexane
6
heptane
7
octane
8
nonane
9
decane
10
H-H eclipsing
0.9kcal/mol
H-CH3 eclipsing
1.4kcal/mol
dihedral angle
chiral
objects that exist as enantiomers
all molecules w/ exactly one stereogenic center are chiral
a molecule w/ no stereogenic center are usually not chiral
OPTICALLY ACTIVE
achiral
molecules contain a plane of symmetry
stereogenic center
only 3° and 4° carbons can be stereogenic
any c with 4 diff substituents
DONT GET FOOLED BY THE RING
drawing enantiomers
draw mirror image
OR
exchange any 2 substituents dash/wedge
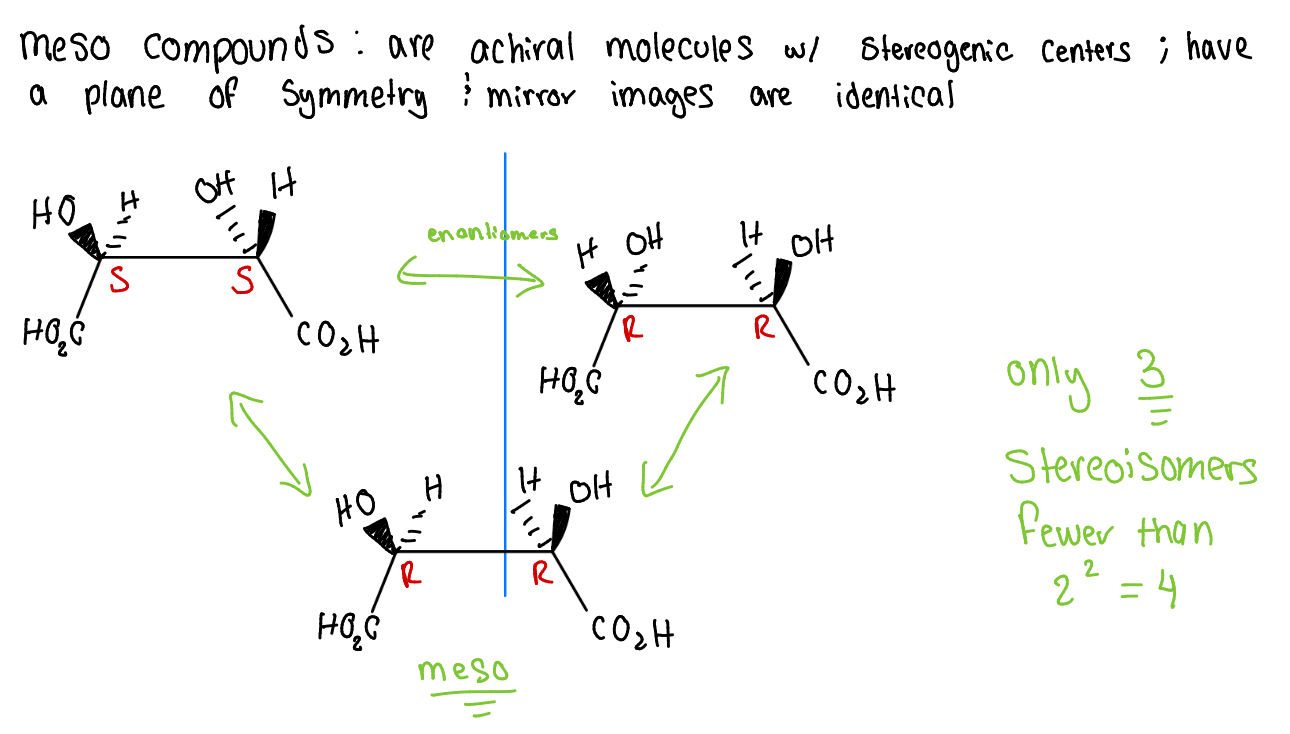
meso compounds
molecules w/ stereogenic centers; have a plane of symmetry & mirror images are identical
mus thave at least two stereogenic centers
racemic mixture
an equal amount of two enantiomers
optically inactive
no rotation (=0°) is observed b/c two enantiomers rotate PPL to an equal extent in opp directions
roation of polarized clockwise vs counterclockwise
clockwise = d or +
counterclockwise = l or -
two enantiomers rotate plane polarized light to an equal extent but in opposite directions
physical properties of isomers
Constitutional isomers and diastereomers have different physical properties.
enantiomers have the same physical properties
can not be separated by physical properties
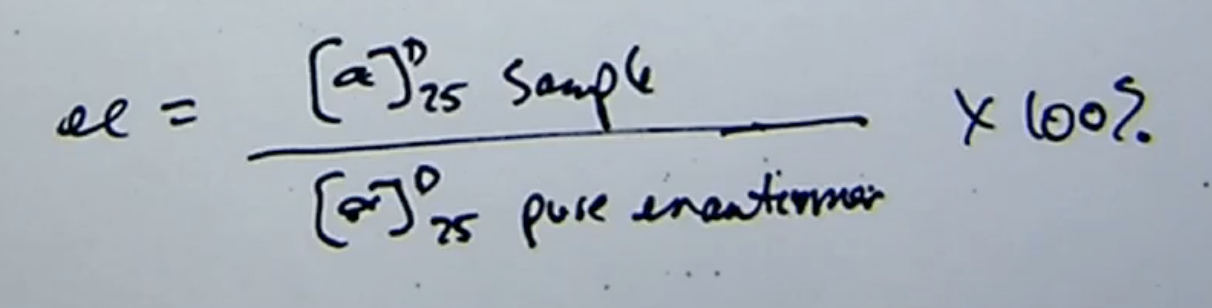
enantiomeric excess