Modern Atomic Theory Test
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Heat vs Temp
Heat: The total energy of molecular motion in a substance (joules/calories)
Temp: the average energy of that motion ( Celsius, Fahrenheit, or Kelvin)
Specific Heat Capacity
A measurement of a materials ability to absorb heat.
Measures the heat energy required to raise the temperature of one unit mass of a substance by one degree Celsius.
Measured in (J/kg°C).
Law of Conservation of Energy
Energy cannot be created or destroyed, only transformed from one form to another.
Light Equation (E=hc/λ)
The equation relates the energy (E) of a photon to its wavelength (λ). Here, h is Planck's constant (6.626 × 10⁻³⁴ J·s), c is the speed of light (3.00 × 10⁸ m/s), and λ is the wavelength in meters. The equation shows that energy is inversely proportional to wavelength: shorter wavelengths have higher energy.
Photoelectric Effect
The phenomenon where electrons are emitted from a material (typically a metal) when it absorbs light of sufficient energy. This effect demonstrates that light can behave as both a wave and a particle (photon), and it provided evidence for the quantum nature of light. The emitted electrons' energy depends on the light's frequency, not its intensity.
Bohr Model
Describes the atom as having a central nucleus with electrons orbiting in fixed paths or energy levels. Electrons can only occupy certain allowed orbits, and they gain or lose energy by jumping between these levels, emitting or absorbing photons.
Bright Line Emission Spectra
Unique patterns of light emitted by atoms when they transition from a higher energy state to a lower one. These serve as a "fingerprint" for identifying elements and understanding their electronic structure.
Limitations of Bohr Model
Cannot explain spectra of multi-electron atoms.
Assumes fixed orbits, neglecting electron wave nature.
Fails to account for electron spin and relativistic effects.
Does not explain the Zeeman and Stark effects.
Inadequate for predicting chemical behavior of atoms.
Limited to hydrogen-like systems only.
Quantum Mechanical Model
describes the behavior of electrons in atoms using quantum mechanics. Replaces the classical notion of fixed orbits with probabilistic electron clouds, where the precise position and momentum of an electron cannot be simultaneously known.
De Broglie
Introduced the idea that every moving particle has an associated wavelength
Heisenberg
Developed the uncertainty principle, stating that certain pairs of physical properties cannot be simultaneously known with arbitrary precision.
Schrödinger
contributed significantly to the understanding of wave functions and superposition in quantum theory.
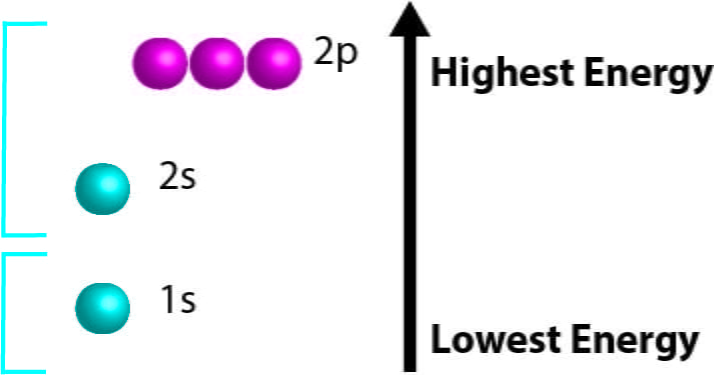
Aufbau Principle
States that electrons occupy the lowest available energy levels first. This principle helps explain the electron configuration of elements, ensuring stability by minimizing energy.
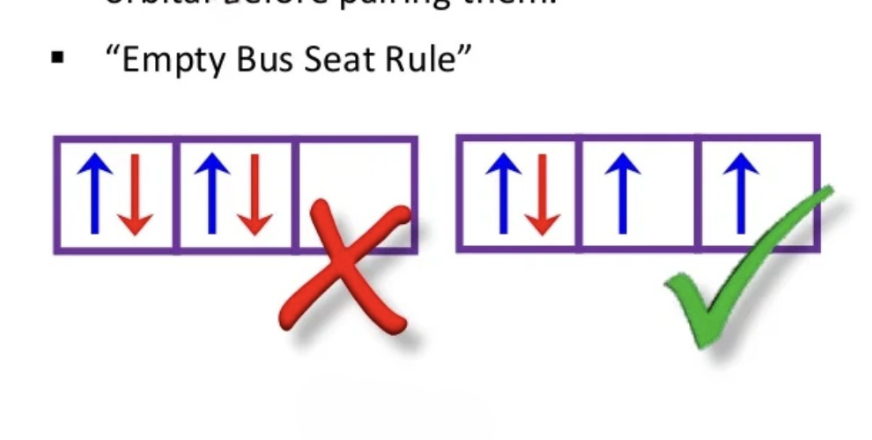
Hund’s Rule
In a given subshell, electrons will occupy empty orbitals singly before pairing up in the same orbital. This minimizes electron-electron repulsion and leads to a more stable arrangement. It is essential for predicting electron configurations in atoms.
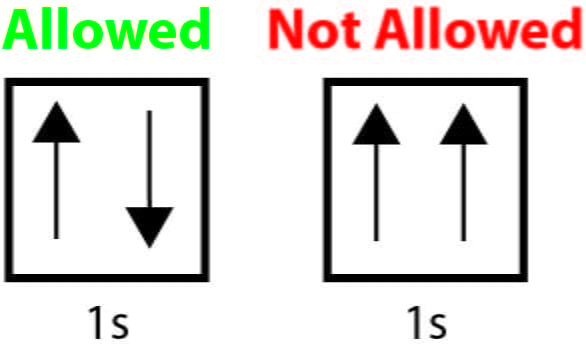
Pauli Exclusion Principle
This principle states that no two electrons can occupy the same quantum state simultaneously within a quantum system.
Electrons that are in the same orbital move opposite directions (CCW/CW)
Kinetic Energy
Energy of motion; depends on mass and speed.
Potential Energy
Stored energy based on position or state; gravitational and elastic are common forms.
Thermal Energy
Energy related to temperature; results from the movement of particles.
Chemical Energy
Energy stored in chemical bonds; released during reactions.
Electrical Energy
Energy from electric charges; powers devices and systems.
Nuclear Energy
Energy stored in atomic nuclei; released during nuclear reactions.
Mechanical Energy
Sum of kinetic and potential energy in an object.
Red
620–750 nm
Orange
590–620 nm
Yellow
570–590 nm
Green
495–570 nm
Blue
450–495 nm
Indigo
425–450 nm
Violet
380–425 nm
s orbital
Circle shape
p orbital
hourglass
d orbital
x shaped