VSEPR Theory
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Valence Shell Electron Pair Repulsion (VSEPR) Theory
Electron groups - lone pairs, single bonds, multiple bonds, single electrons - repel one another through coulombic forces.
- electron groups attracted to nucleus
- maximum separation = best
Linear Geometry
- 2 electron groups (2 single bonds)
- 180 degree bond angle
- EX: CO2
Trigonal Planar Geometry
- 3 electron groups
- 120 degree bond angles
- EX: BF3
Tetrahedral Geometry
- 4 electron groups
- three dimensional
- 109.5 degree bond angles
- EX: CH4, methane
Trigonal Bipyramidal Geometry
- 5 electron groups
- 3 groups on flat plane (120 degrees)
- 2 above/below axial positions (90 degrees)
- Angles differentiate
- EX: PCl5
Octahedral Geometry
- 6 electron groups
- 4 groups lie on flat plane
- 2 above/below axial positions
- All angles are 90 degrees
electron geometry
the geometrical arrangement of the ELECTRON groups
molecular geometry
the geometrical arrangement of the atoms
bent molecular geometry
- trigonal planar derivative
- 120 degree bond angles
- 2 bonded atoms, 1 or 2 lone pairs
trigonal pyramidal molecular geometry
- tetrahedral derivative
- 4 electron groups
- 1 lone pair of electrons
- 3 bonding pairs
- less than 109.5 degree bond angles
- EX: NH3 107 degree
effect of lone pairs on molecular geometry
the more lone pairs, the more repulsion, the smaller bond angle
see-saw molecular geometry
- trigonal bipyramidal derivative
- 5 electron groups
- 4 bonding pairs, 1 lone pair
- 2 90 degree bond pair repulsions
- EX: SF4
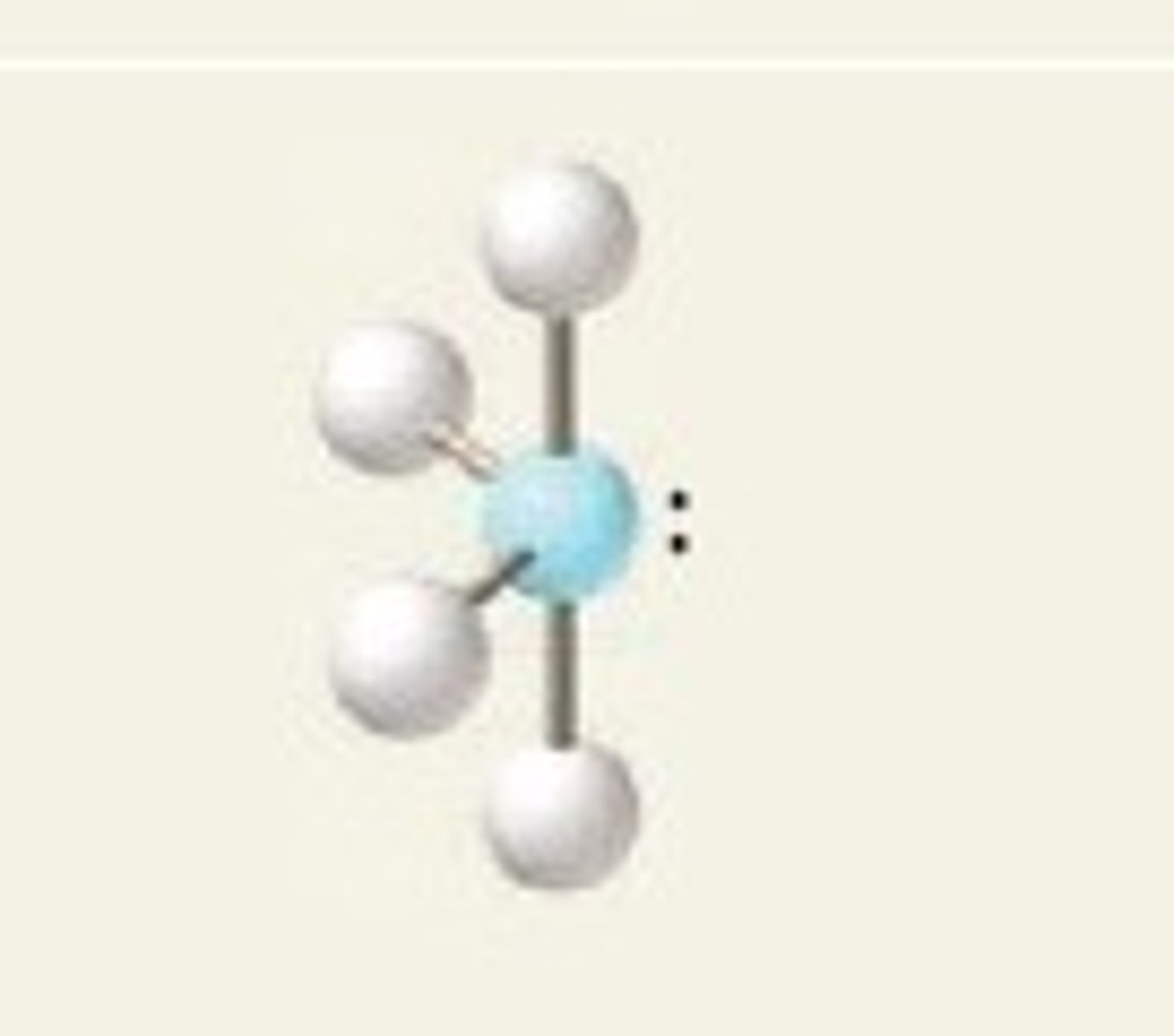
T-Shaped molecular geometry (5)
- trigonal bipyramidal derivative
- 5 electron groups
- 3 bonding pairs, 2 lone pairs
- bond angles 90 degrees
- EX: BrF3
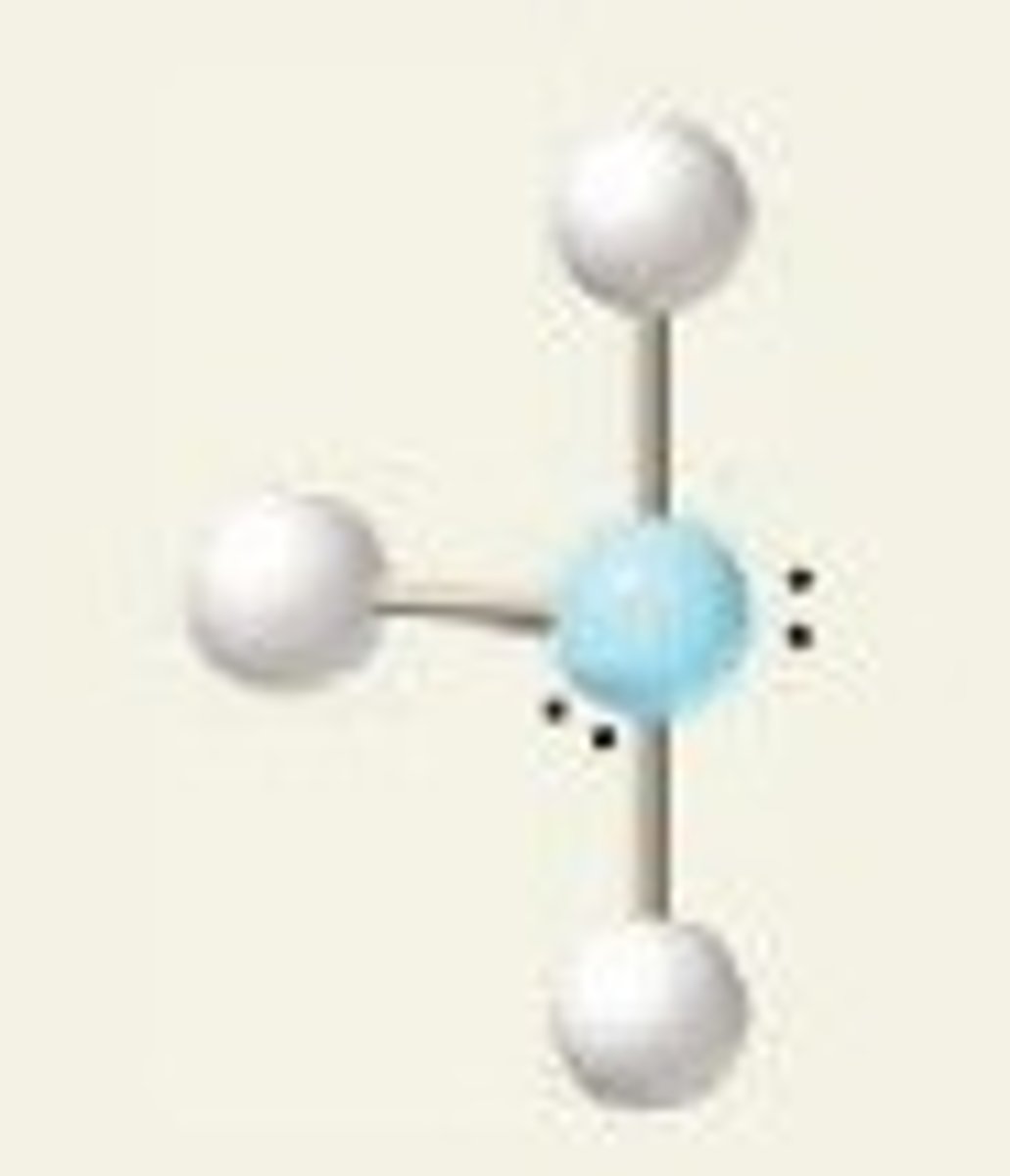
Linear molecular geometry
- trigonal bipyramidal derivative
- 5 electron groups
- 2 bonding, 3 lone pairs
- 180 degrees
- E: XeF2
Square Pyramidal molecular geometry
- octahedral derivative
- 5 bonds
- one lone pair
- 90 degree bond angles
Square Planar molecular geometry
- octahedral derivative
- 4 bonds
- 2 lone pairs
- 90 degree bond angles
T-Shaped molecular geometry (6)
- octahedral derivative
- 3 bonds
- 3 lone pairs
- 90 degree bond angles
Linear molecular geometry (5)
- trigonal bipyramidal derivative
- 2 bonding, 3 lone pairs
- 180 degree bond angles