Acids and Bases
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
Acids taste
sour

Bases taste
bland/ bitter


Acids turn litmus paper
red
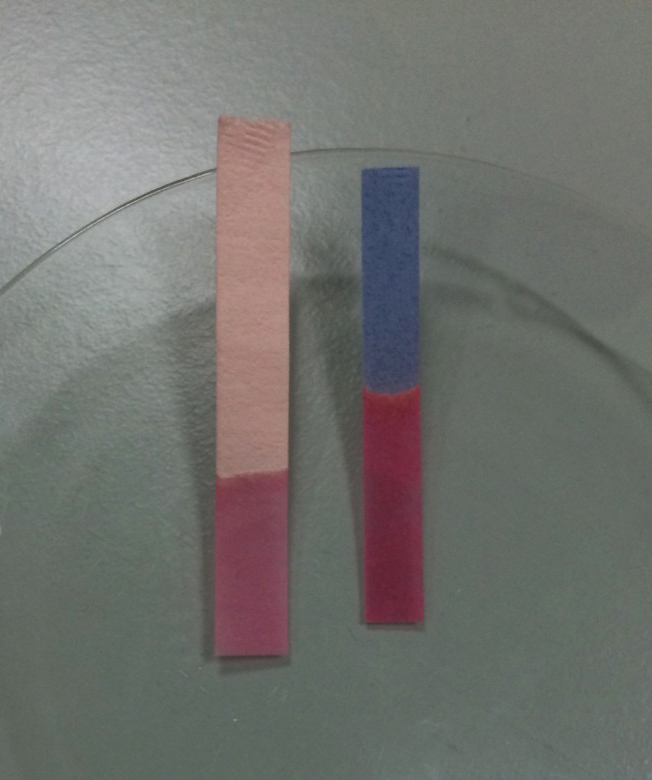

Bases turn litmus paper
blue

acids feel
irritating to the skin and sticky
bases feel
slippery
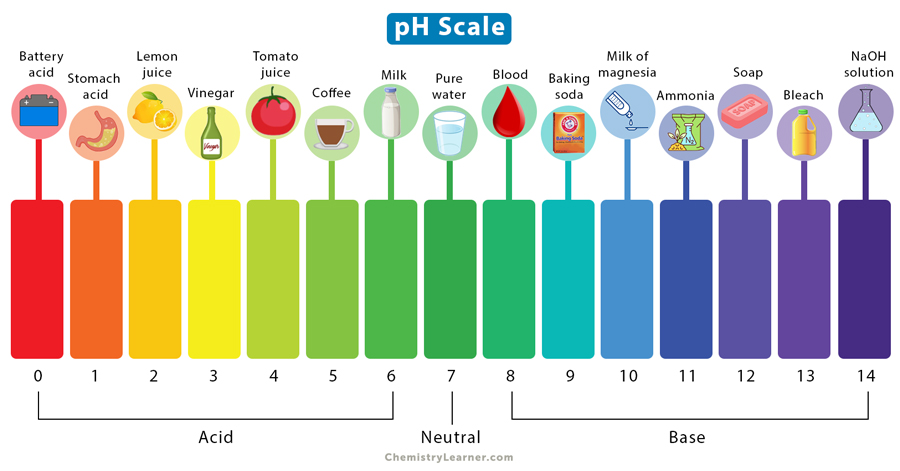
whats the pH range of acids?
low (< 7)
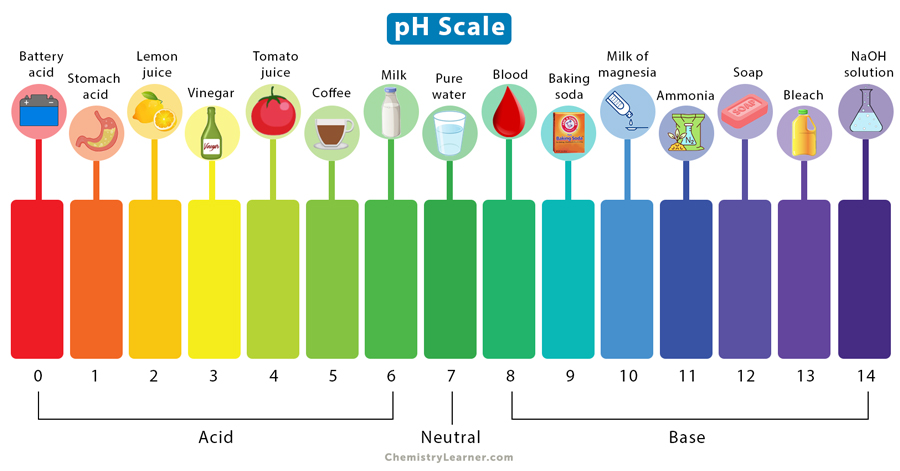
whats the pH range of bases?
high (> 7)
what is the characteristic group of acids?
H⁺ ion
what is the characteristic group of bases?
OH⁻
Are acids a strong or weak conductor?
strong conductor in aqueous solutions

Are bases a strong or weak electrolytes?
can be strong or weak
Acids ________ with metals
react
Bases __________ with metals
dont react
Arrhenius theory on acid
acids produce nitrogen ions (H⁺) in aqueous solutions
Arrhenius theory on bases
bases produce hydroxide ions (OH⁻) when dissolved in water
Lewis theory on acids
acids are the electron pair acceptor
Lewis theory on bases
bases are the electron pair donor
Bronsted theory on acids
is hydrogen-ion donor (H⁺ or proton)
Bronsted theory on bases
base hydrogen- ion acceptor
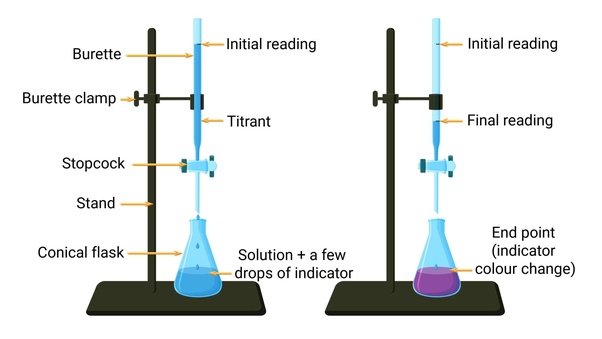
What is a titrant?
what is dropped into the titration (the base)
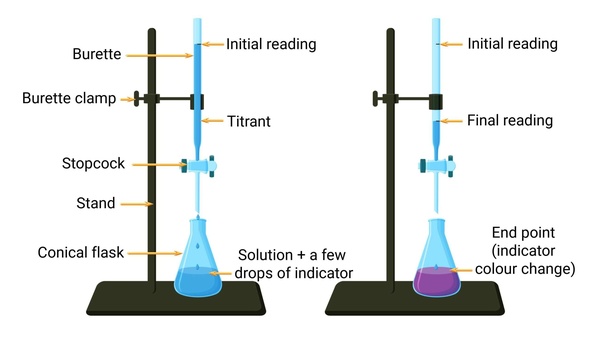
What is an end point?
when the color changes in a titration
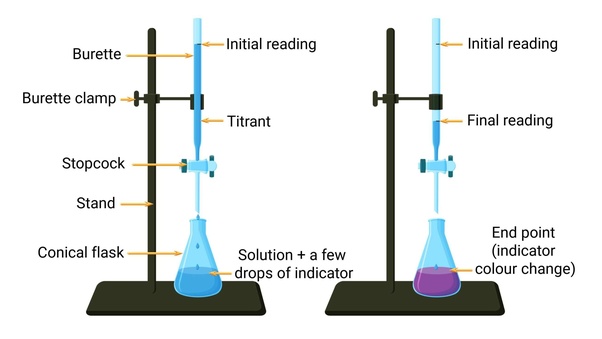
What is a standard solution?
what is known
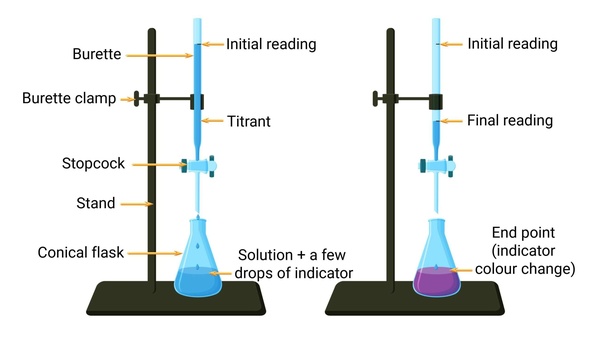
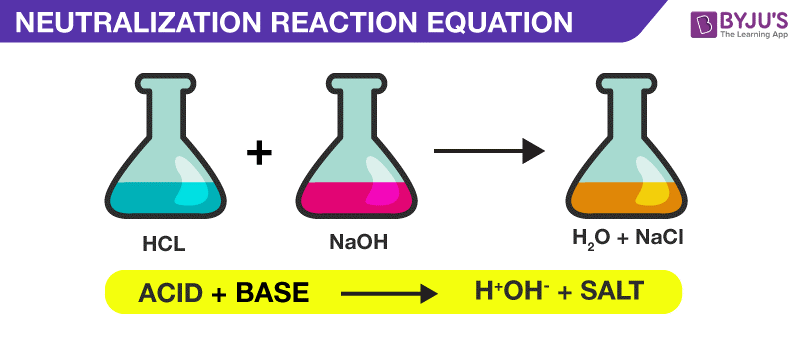
What is neutralization?
a reaction in which and acid and a base react in an aqueous solution to produce a salt and water
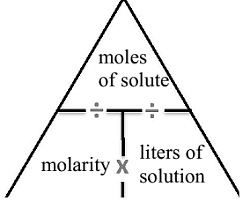
What is molarity?
a unit of concentration expressed as the number of moles of dissolved solute per liter of solution.

How do you find the end point of a titration?
you read the burette after the color of the solution changes

How do you calculate the end point?
initial reading - final reading = endpoint
How do you complete a titration?
Rinse the burette thoroughly with distilled water
pour about 10mL of titrant and roll it around the burette to clean it open stopcock and rinse when done
fill burette with the titrant slightly past the 0.0mL mark and drain it to exactly 0.0mL and record the initial reading
prepare the solution to titrate in an Erlynmeyer flask with 2-3 drops of indicator and swirl the solution
begin titrating by slowly opening and closing the stopcock and swirling the solution until the solution becomes a light pink
Record he final reading and subtract from the initial
pOH=
-log [ OH- ]
pH + pOH =
14
[ H⁺ ] =
10⁻ᵖʰ
[ OH- ] =
10⁻ᵖᴼᴴ
pH stands for
power of hydrogen
