Chap 14A - Alkenes
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Describe nonemclature of alkenes
Identity longest continuous C chain containing C=C bond
Identify the position of the C=C bond
Number the carbon atoms consecutively from the end of the parent chain which gives the lower number for the position of the C=C bond
Identify the alkyl substituent(s) (CnH2n+1) attached to the parent chain and indicate the position(s) of the substituent(s)
Arrange the substituents in alphabetical order, ignoring the prefixes such as di– or tri–
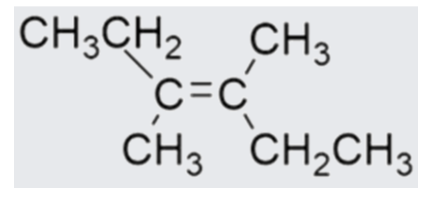
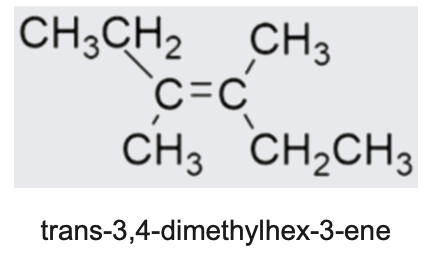
State name of this structure
Describe test for alkenes (reagents, conditions)
Reagent and condition: Br2 in CCl4 or Br2 in H2O (aqueous Br2)
Observation: Decolourisation of a solution of orange–red Br2 in CCl4 or orange Br2 in H2O
A good test for an organic compound should give an observable visual result where
there is:
Colour change
Precipitate formed and the colour of the precipitate
Gas given off and identify the gas
Describe preparation of alkenes from dehydration of alcohols (type of reaction, reagents, conditions)
Type of reaction: Elimination
OH group and H atom from 2 adjacent C atoms are eliminated -> C=C bond formed with H2O as by-product
Reagents & conditions: concentrated H3PO4 catalyst + heat, excess concentrated H2SO4, heat OR Al2O3 + heat
Describe preparation of alkenes from Dehydrohalogenation of Halogenoalkanes (type of reaction, reagents, conditions)
Type of reaction: Elimination
Reagents & conditions: NaOH in ethanol + heat
This is an acid-base reaction
Describe the products from Dehydrohalogenation of Halogenoalkanes (explain why one is the major product)
When an unsymmetrical halogenoalkane (Eg. (CH3)2CHCH(Br)(CH3) heats with NaOH in ethanol, there are two products formed)
The major product formed is the more stable and substituted product
The more substituted alkene is the one with more alkyl groups (CnH2n+1) bonded to the C atoms in the C=C bond
The enthalpy change of hydrogenation of the more substituted alkene to form the corresponding alkane is found to be less exothermic than its isomeric alkene which is less substituted
Describe solubility and density of alkenes
Solubility and Density |
|
Describe mp/bp of alkenes (number of C atoms and branched VS straight)
Number of C atoms
Boiling point increases with number of carbon atoms as more energy is required to overcome the stronger id-id interactions due to increasing number of electrons
Branched VS straight
Branched chain isomers have lower boiling points than their straight chain isomers
Branched chain isomers are more spherical with less surface area of contact between molecules for electron interactions -> weaker id-id interactions
Describe mp/bp of alkenes (cis-trans isomer)
Cis isomer has larger net dipole moment -> it is a polar molecule and is held by stronger pd-pd interactions and has a higher boiling point than the non–polar trans isomer
Since the dipole moments in the trans-isomer are equal and opposite in direction, the dipole moments cancel out and net dipole moment becomes zero
In the liquid state, the molecules are not rigidly held in fixed positions and they are not very close together as in the solid state -> packing of molecules in trans isomer is not influential to account for differences in boiling points
Trans isomer has a higher melting point than the cis isomer due to the closer packing of the trans isomer molecules
Explain why in C=C, strong sigma and weak pi
Strong sigma bond and weak pi bond
Since the π electrons are much more exposed than those in the σ bond, this makes the π bond a good source of electrons and the C=C bond is also a region of high electron density -> alkenes are more vulnerable to be attacked by electrophiles
Typical reaction of alkenes is electrophilic addition
Describe addition of hydrogen halides (general reaction, type of reaction, reagents, conditions)
Addition of hydrogen halides
Type of reaction: Electrophilic addition
Reagent and conditions: HX(g)
Describe mechanism of addition of hydrogen halides for symmetrical alkenes
For symmetrical alkenes |
|
Describe addition of hydrogen halides for unsymmetrical alkenes (mechanism for propene)
For unsymmetrical alkenes |
|
Use marknovinikovs rule to identity major product formed from addition of hydrogen halides for unsymmetrical alkenes
Marknovnikov’s Rule
Used to identity which is the major product
The major product of the electrophilic addition reaction will be the one formed from the more stable carbocation intermediate
Stability of carbocation: tertiary > secondary > primary
Tertiary carbocation is the most stable as it has three electron donating alkyl (R) groups, which exert electron donating inductive effect to help disperse the positive charge on carbocation to a greater extent