BIO315 - Cell Bio
1/75
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
Cell communication
allows organisms to respond to changes in their environment
Intracellular signalling/signal transduction
A signal molecule(ligand) attaches to a receptor which sends a signalling cascade to alter the effector(target protein) and alters the cell behaviour
4 components of cell communication
Signal molecules
Receptor proteins
Intracellular signalling proteins
target/effector proteins
Extracellular signalling can…
can act over short and long distances
4 Classes of intracellular signalling
contact-dependant, paracrine, endocrine, synaptic
Contact-dependant signalling
cell-to-cell by membrane-bound ligands
Paracrine signal
The ligand secreted by the cell in the ECF to target cells in the near vicinity
Endocrine signal
hormone secreted into the bloodstream and travels to target cell/receptor -> takes longer
Synaptic signal
neurons connected to other neurons/target cells
Receptor Binding types
cell surface -> on the cell membrane
intracellular receptors -> within the cell
A combination of biological responses in a cell can…
have many receptor types and knowing when to respond to a signal depends on the signal type, the concentration of the signal, or the absence of a signal
Cell types and responses
Responses depend on the cell type the signal is given to ex. Acth on heart cells will decrease the firing rate of signal
Signal concentrations are important since…
Some cells need higher or lower concentrations of a signal to be activated -> varies with the cell
3 classes of cell surface receptors
ion-channel, G-protein, enzyme-coupled
Ion-channel coupled receptor
transmit by ion-gated channels and a neurotransmitter binds to the channel and causes it to close/open -> excitability of a cell
G-protein receptor
by heterotrimeric G proteins to regulate membrane-bound target proteins
Enzyme coupled receptor
receptor has enzymatic activity or uses an enzyme to activate the receptor -> usually has protein kinase activity
Second Messengers
relay signals from the membrane-bound proteins to effector proteins -> like on/off switches on proteins → relay signals
Importance of intracellular signal proteins
relay signals, act as a scaffold, spread signal from one pathway to another
Phosphorylation/dephosphorylation
the addition or elimination of a phosphoryl group to a molecule - storage/transfer of free E
Signal by phosphorylation
Signal in -> protein kinase uses ATP -> signalling protein on ->
Signal by dephosphorylation
protein phosphatase removes Pi from ATP -> signalling protein off
Kinase cascade
(multiple protein kinases working - in sequences) one gets activated, and it activates the next, in a signal line -> adds more phosphate groups
Protein kinase vs phosphatase
Protein kinase adds a phosphate while protein phosphatase remove phosphate group
3 Types of kinases
ser/thr -> phosphorylate the hydroxyl groups in ser/thr
Tyr -> phosphorylates proteins on tyr
dual specificity -> can phosphorylate on all three ser/thr/tyr
GTP/GDP binding signalling
Causes a change inside the cell from being outside the cell -> on when GTP bound, off when GDP bound
When on, they have GTP activity and shut off when hydrolyzing their GTP to GDP
Types of GTP proteins
heterotrimeric G protein -> help relay signals from G-protein receptors -> many subunits
monomeric GTPases -> help relay signals from many cell-surface receptors -> one subunit
Regulation of monomeric GTPases
GAP, GEF, and GDI
GAP GTPase
causes the off state by hydrolyzing more GTP - once the G protein is bound to GTP, it will be hydrolyzed to return to the inactive state
GEF GTPase
causes the on state by eliminating GDP and allowing more GTP to bind - release GDP and bond GTP
GDI
binds to G protein in the off-state and prevents the release of GDP -> promotes the off-state
Inhibition steps of signalling
Most signals have activation and inhibition steps
Two inhibitory effects in one path can create a positive effect
Signal -> protein kinase -> inhibitory protein -> transcription regulator free -> induce gene regulation
How to ensure signal response and specificity?
High-affinity interactions that are highly specific, Signal threshold, Localization
Localizing by scaffold protein
use of specific binding sites - brings groups of interacting signalling proteins into signalling complexes
Localizing at the site of unactivated receptors
in the scaffold protein that is inactive but proteins are inserted already, they are activated in a downstream manner when the signal protein activates the receptor
Localizing by phospholipids
docking on the membrane and interacting with other signalling proteins when the receptor on the membrane is activated
The head group attracts the proteins to bind t
Importance of interaction domians
a small region of a protein that has the correct structure to bind to a specific motif of another protein
Induced proximity
signal triggers the assembly of complex proteins by bringing proteins closer together -> triggers the protein to come closer together
interaction domains
-> areas of the proteins where they bind to other proteins
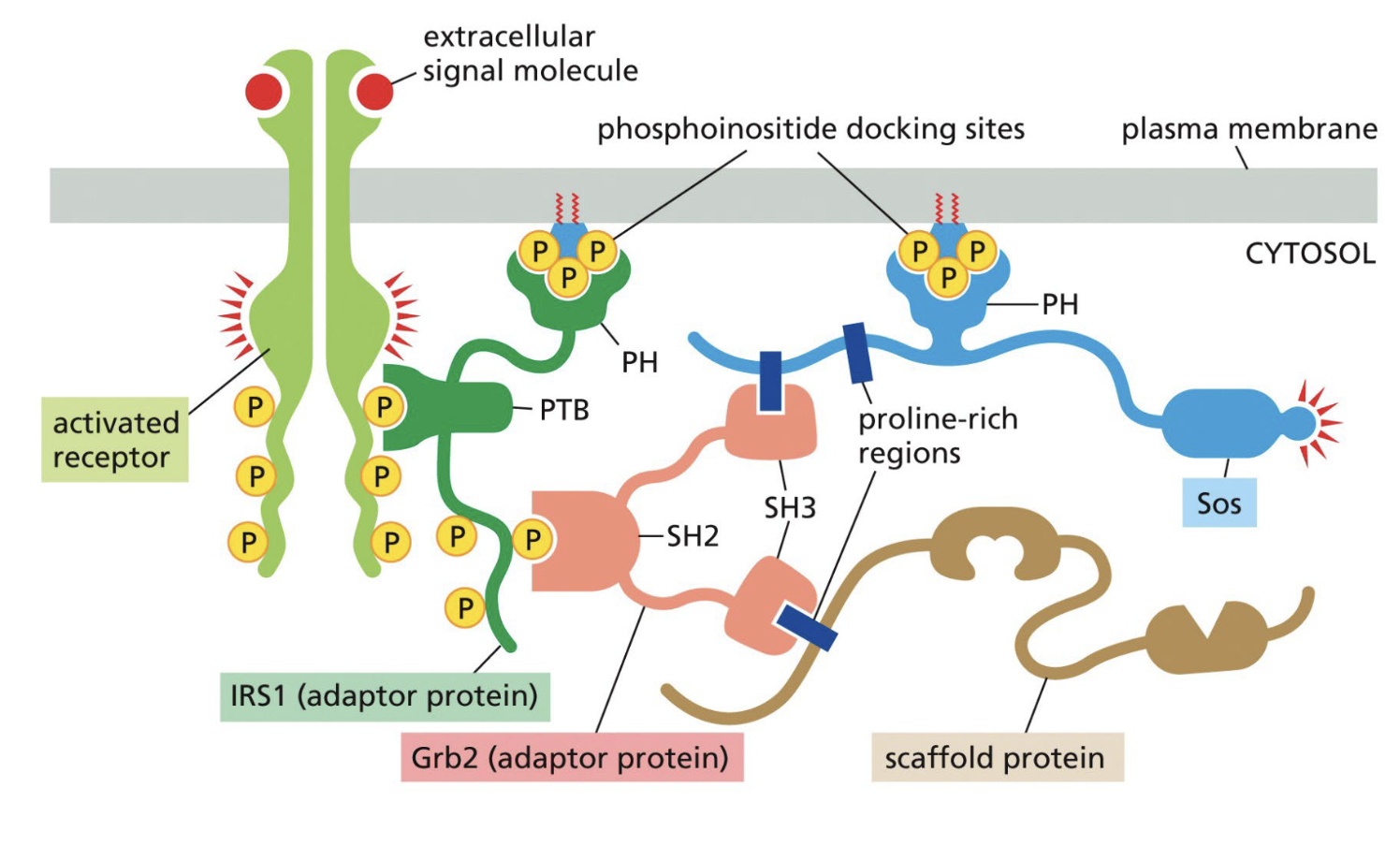
Signal complex in Insulin receptor
Different types of signalling pathway properties
Response time(synaptic = fast, endocrine = slow)
sensitivity(receptors, some need lower concentrations of a signal to activate, others higher[C]) -> some extremely sensitive, some need more signal molecule
dynamic range(adaptation mechanisms, some need specific signals other can have broader ones) - range a pathway reacts to(narrow or broad)
persistence(+/- feedback) - how long a response lasts(some longer lasting)
signal processing(ex. Gradual to abrupt) - simple signal to complex response
integration(many signals can activate one signal)-> need coincidence detectors
coordination(one signal to a response that activates many signals)
Signal integration/coincidence detectors
Combinatorial signals -> helped by coincidence detectors
Senses when two signals come in at the same time and allow the cell to decide on an appropriate response
Factors influencing response times
Depends on what needs to be done -> depends on what the effector protein is and what the end outcome is
Turnover in signalling molecules
inhibition processes determine how quickly and great the response can be -> activation needs to be quickly revered to make rapid signalling possible
Half-life in signal molecules
Very stable at steady state, they are built up, if you start low, you will take a lower time to double than if you started higher, and will take longer
Short-half life is unstable since it can rapidly increase in amount and response time at initiation is very quick
Short-half life at termination will drop very quickly than a molecule than that of a longer half-life molecule
Signal processing in biological processes
Some signals need slower responses, like hormones, to be able to fine-tune their signals
Other systems need faster signalling, like action potentials, to work right -> all-or-one response is maximal
Allosteric Binding
to make the response more steep, you can incorporate signalling proteins that are controlled by allosteric bonding - very high concentration does this happen
-/+ feedback
- stops the signal from countiuing
+ continues the signal more and more
Negative feedback
allows cells to use a small [C] of signals to do what they need
Positive feedback
gradual increase in [C] that gets more and more, but it will eventually stop -> all-or-none reactions
Regulating signal sensitivity and dynamic range
Adaptation!
Adaptation
the ability of a cell to respond to a wide range of signals [C]
GPCR
G-Protein Coupled Receptor
one ligand can bind to many GPCRs and one GCPR to many ligand types - all are 7 transmembrane and signals through heterotrimeric G proteins
Heterotrimeric G-proteins
all have 3 subunits of alpha, beta, and gamma
In an unstimulated state, alpha has GDP bound and G protein inactive
GPCR is active(performs GEF activity), alpha releases GDP to bind to GTP -> GTP causes a conformational change that releases G protein from receptor and dissociation of alpha G from beta and gamma pair G - they then can relay signals onwards
What happens to the alpha subunit when it becomes unstimulated?
Alpha then hydrolyzes the GTP to GDP and becomes inactive - bound to GDP
What do G-coupled receptors do?
Biological functions include smell and taste, light perception, neurotransmission, endocrine/exocrine glands, control of BP, exocytosis, cell growth and development, etc
Activation of heterotrimeric G-proteins act like…
GEF in GPCRs
What is cAMP?
cAMP acts like a second messenger in some systems and are made from ATP by adenylyl cyclase and destroyed by cyclic AMP phosphodiesterases
G-proteins and cAMP
The alpha subunit in G proteins stimulates cAMP synthesis
Gi proteins(inhibitory G protein) inhibit the cAMP synthesis
Cholera toxin
inhibits the switch-off mechanism of Gs and allows elevated cAMP [C] since the alpha unit is GTP bound and on
Pertussis toxin
inhibits the switch on of Gi that prevents the protein from interacting with others and keeps it in its inactive state
Remember that Gi is…
DIFFERENT FROM Gs!
Cellular responses mediated by cAMP
Thyroid gland
Adrenal cortex
Ovary
Muscle
Bone
Heart
Liver
Kidney
Fat
cAMP dependant protein kinase
cAMP activates PKA that phosphorylates specific serines or threonines and regulates signal protein activity
Inactive PKA has two catalytic subunits, cAMP binds and alters their shape and the two release from each other and go to target other proteins
Regulatory proteins help localize the PKA to a specific complex via A-kinase anchoring proteins(AKAPs)
CREB(CRE-binding protein)
cAMP-dependent transcription factor
Recognizes the cis-regulatory sequence called CRE found on many regulatory proteins activated by cAMP
PKA activated, phosphorylates CREB which then recruits a transcriptional coactivator called CBP that stimulates the transcription of target genes
G proteins and phospholipid signalling
activate the plasma-membrane-bound enzyme phospholipase-C(PLCβ) - it activates by Gq G protein that cleaves PI4,5P2 to make two products: IP3 and diacylglycerol
IP3 goes through the ER and opens IP3-gated Ca channels, raising Ca ion[C] in the cytosol
Diacylglycerol activates protein kinase C(PKC) is Ca-dependent and phosphorylates different proteins(similar to PKA)
Gq proteins activate PLCβ and calcium signalling
Phospholipase C(PLC) is activated by Gq -> inositol phospholipid signalling path
PLC is activated by Gq which activates PI4,5P2 and makes two products
Protein Kinase C(PKC)
lacks Ca binding sites and needs to be altered to use Ca
When Ca is increased initially by IP3, it alters PKC to face the plasma membrane where it is activated by Ca, DAG, and negative serine to target other proteins
Diacylglycerol(DAG)
It can be further cleaved to make arachidonic acid that can be used to synthesize prostaglandins or function as a signal molecule -> used in pain and inflammatory responses
Ca as a ubiquitous intracellular mediator
Many extracellular responses raise intracellular Ca[C] -> normally it is lower intracellular than extracellular
Some processes outside the cell raise Ca[C] in the cell while some intracellular processes like IP3 receptors(GPCR mediated signals) increase Ca from inside the cell
Ryanodine receptors
in the ER membrane are activated by Ca and amplify Ca signals
Terminating the Ca2+ signal and maintaining low resting Ca
There are also Ca pumps in the ER and plasma membranes, which use ATP, that return calcium to intracellular stores
Calcium signalling -> spikes, puffs, and waves
IP3 and ryanodine are stimulated by low Ca[C] - Ca-induced calcium release is positive feedback -> a spark when IP3 is stimulated and Ca enters that then activates the next receptor and the next and so on, this sends a wave of Ca when the [C] of Ca is enough to activate nearby receptors and moves through the cytosol -> high [C] across the entire cell(rather than becoming more dilute) -> positive feedback
+/- feedback generates calcium waves
Eventually, the large [C] of Ca shuts down IP3 and ryanodine receptors that shut down Ca release and prevent more Ca from coming in -> Negative feedback -> Ca pumps remove the Ca
Ca oscillations of -/+ feedback
Eventually this (-) feedback will wear out and IP3 will trigger another wave of Ca -> these oscillations will continue to happen -> frequency reflects extracellular stimuli
Ca [C] in the cell and different systems
Different systems can use different amounts of Ca[C] to do different things - some need higher [C] to make one thing or lower [C] to make another -> depending on their sensitivity