Chemistry VESPR Geometry (Linear, Trigonal planar, Tetrahedral)
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
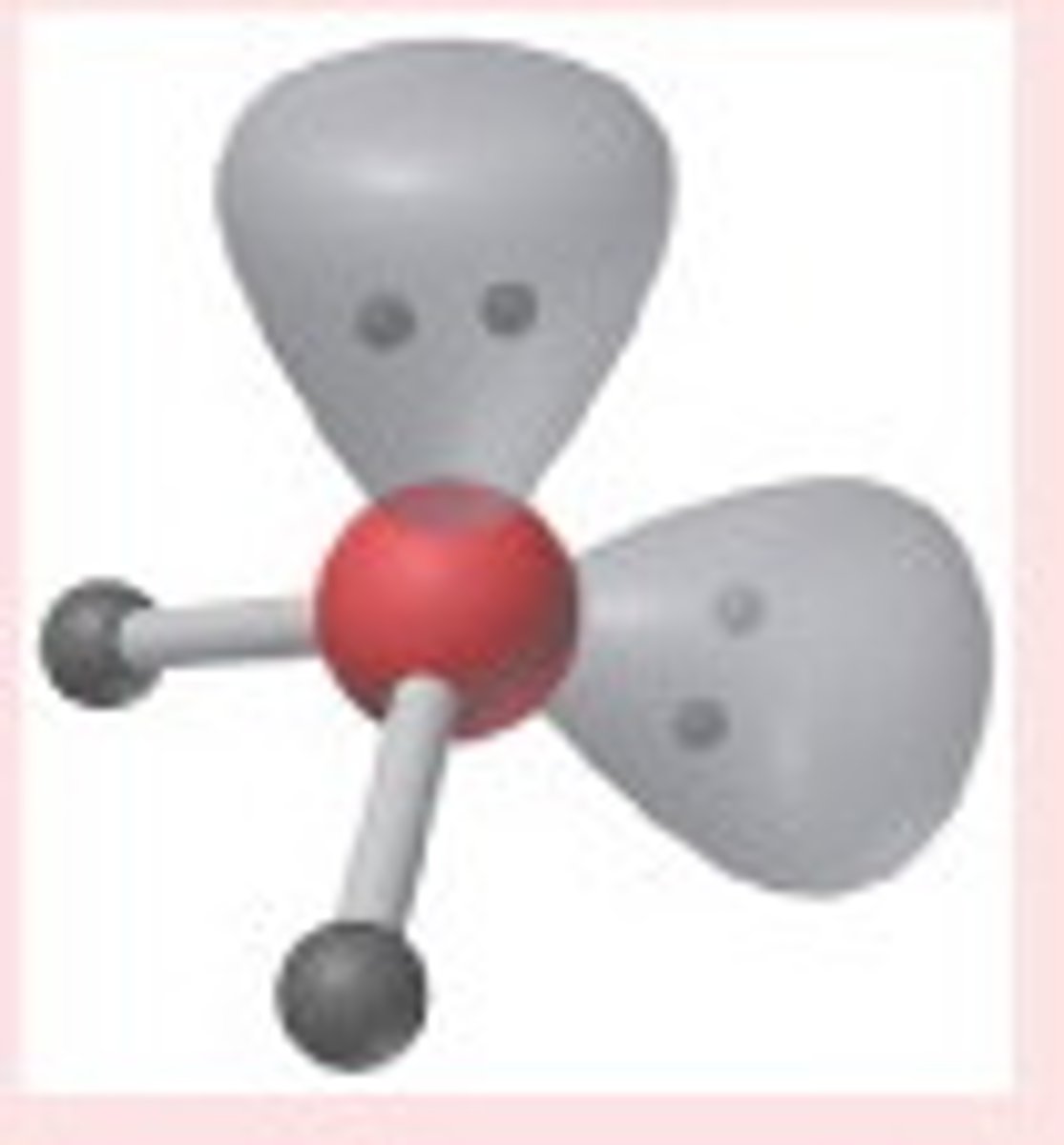
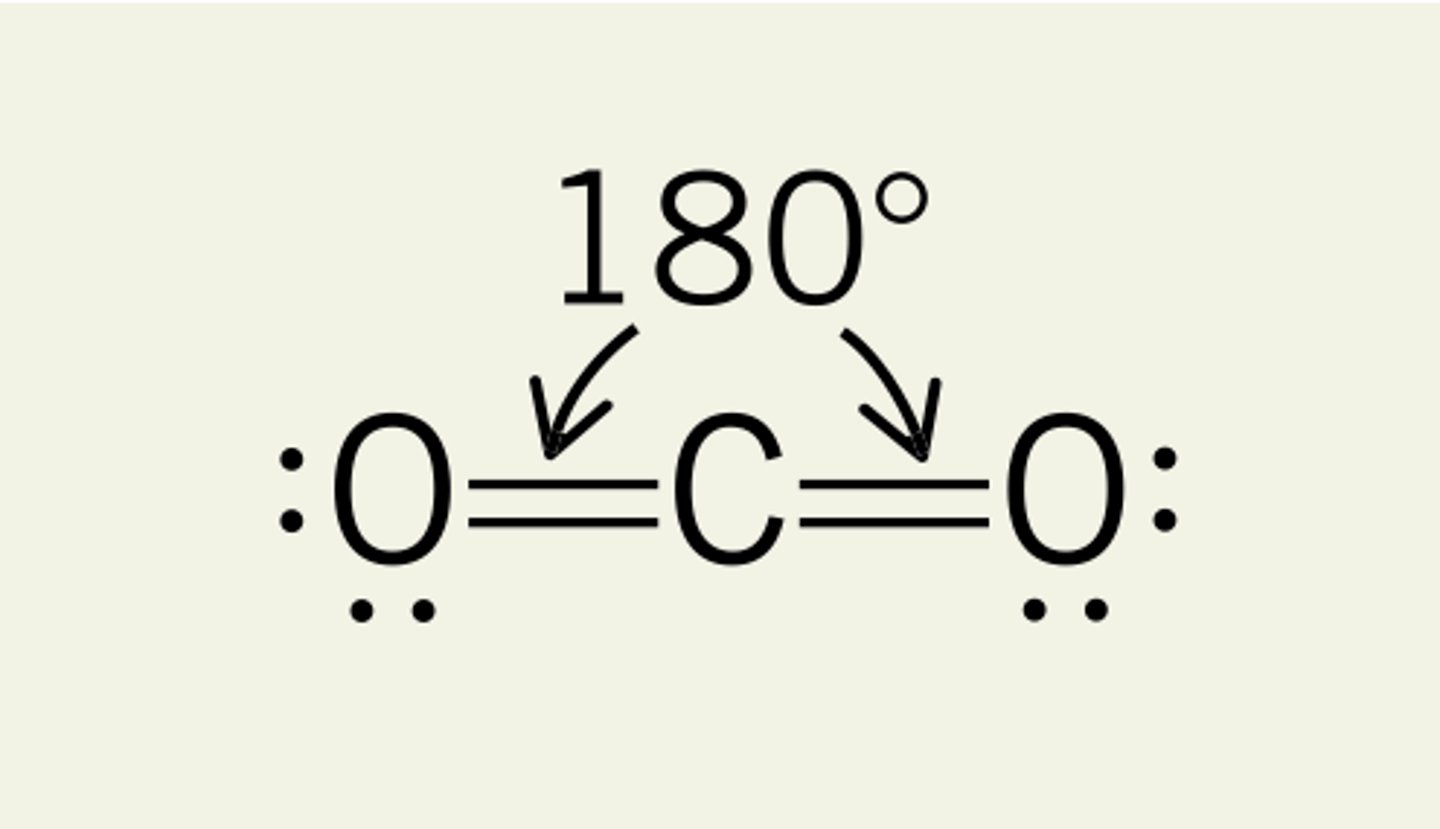
Linear
# of Bonding Groups = 2
# of lone pairs = 0
Approximate Bond Angle = 180
Example = CO2
2
New cards
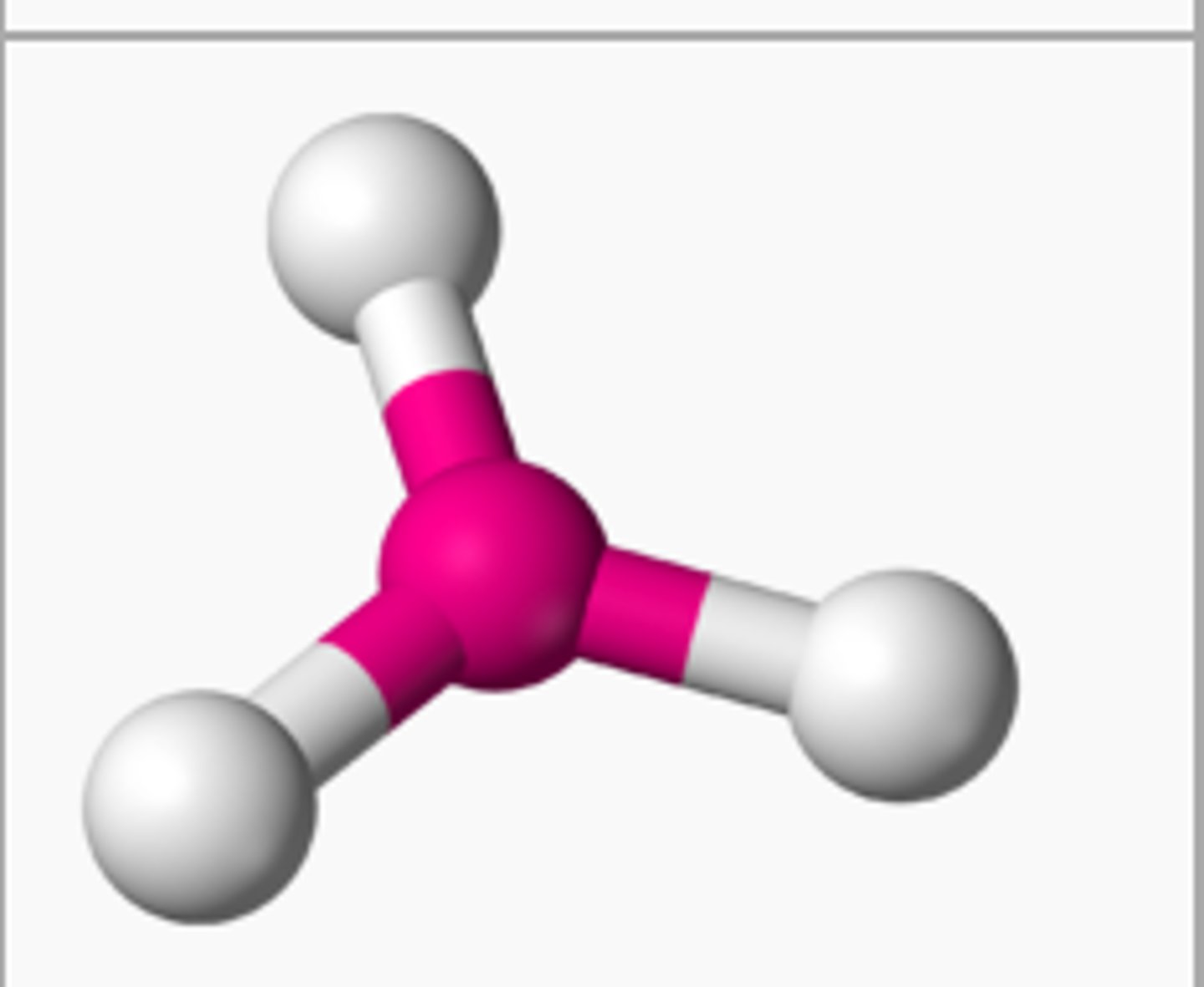
Trigonal Planar
# of Bonding Groups = 3
# of lone pairs = 0
Approximate Bond Angle = 120
Example = BH3
3
New cards
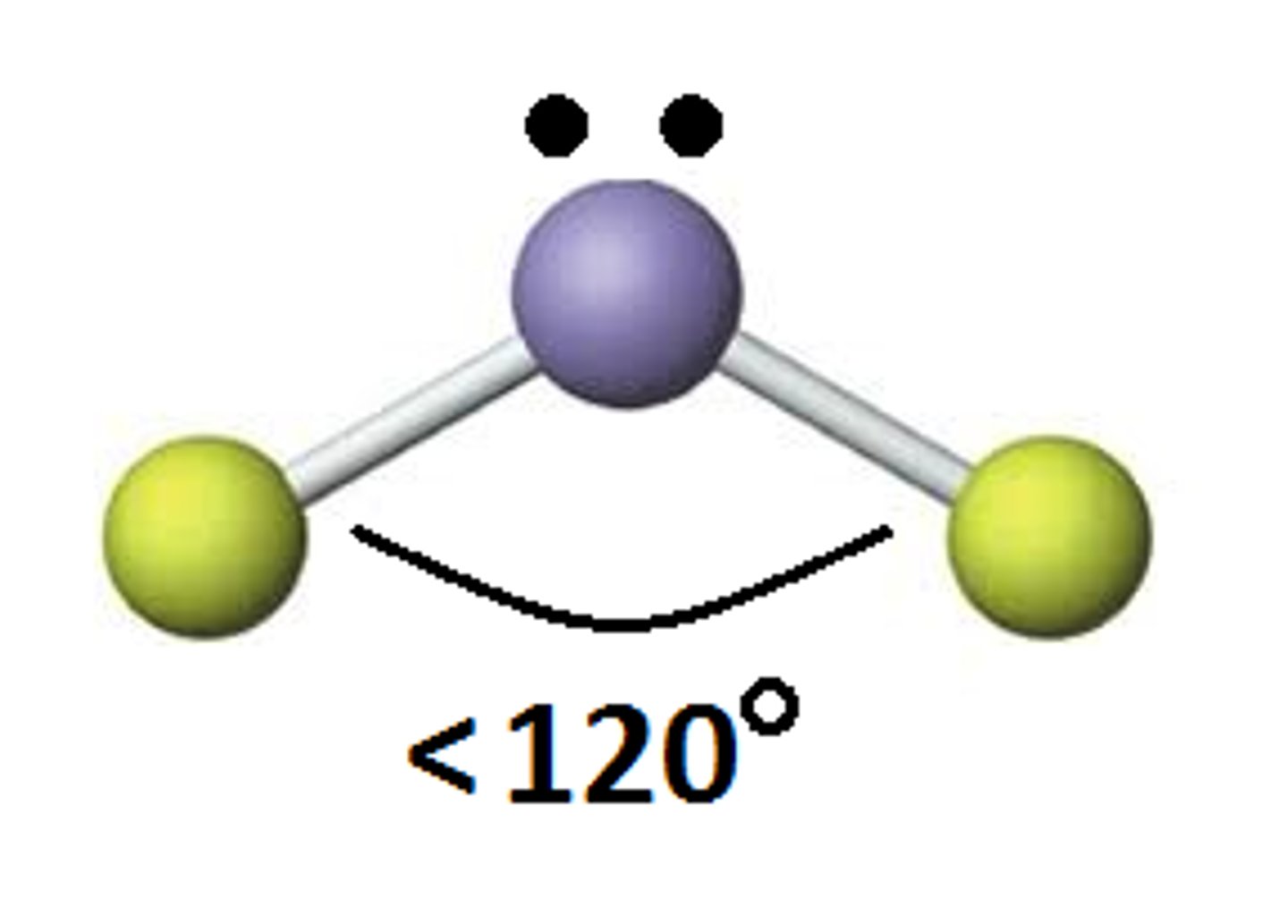
Bent (Trigonal Planar)
# of Bonding Groups = 2
# of lone pairs = 1
Approximate Bond Angle = <120
Example = NO2-
4
New cards
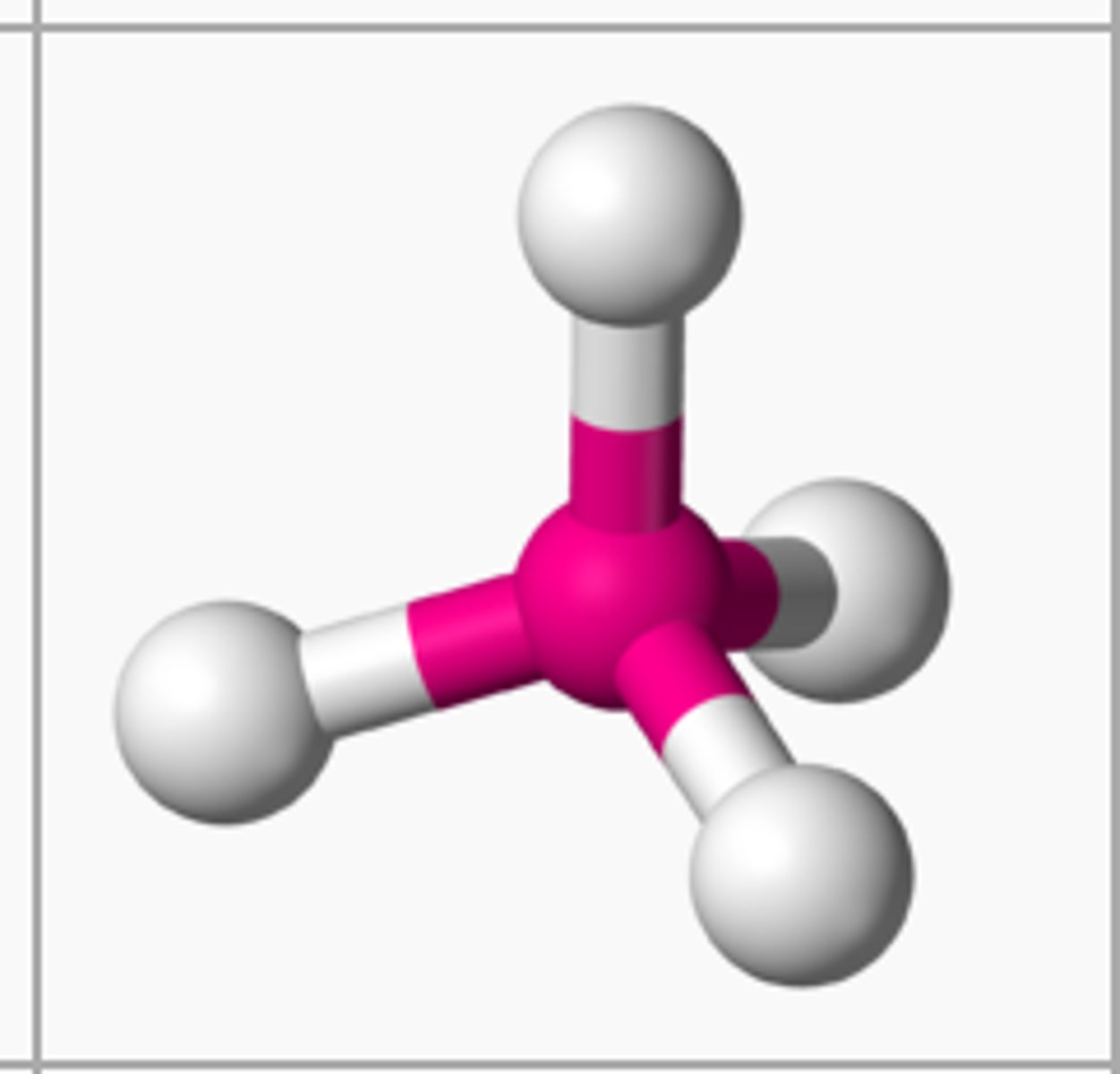
Tetrahedral
# of Bonding Groups = 4
# of lone pairs = 0
Approximate Bond Angle = 109.5
Example = CH4
5
New cards
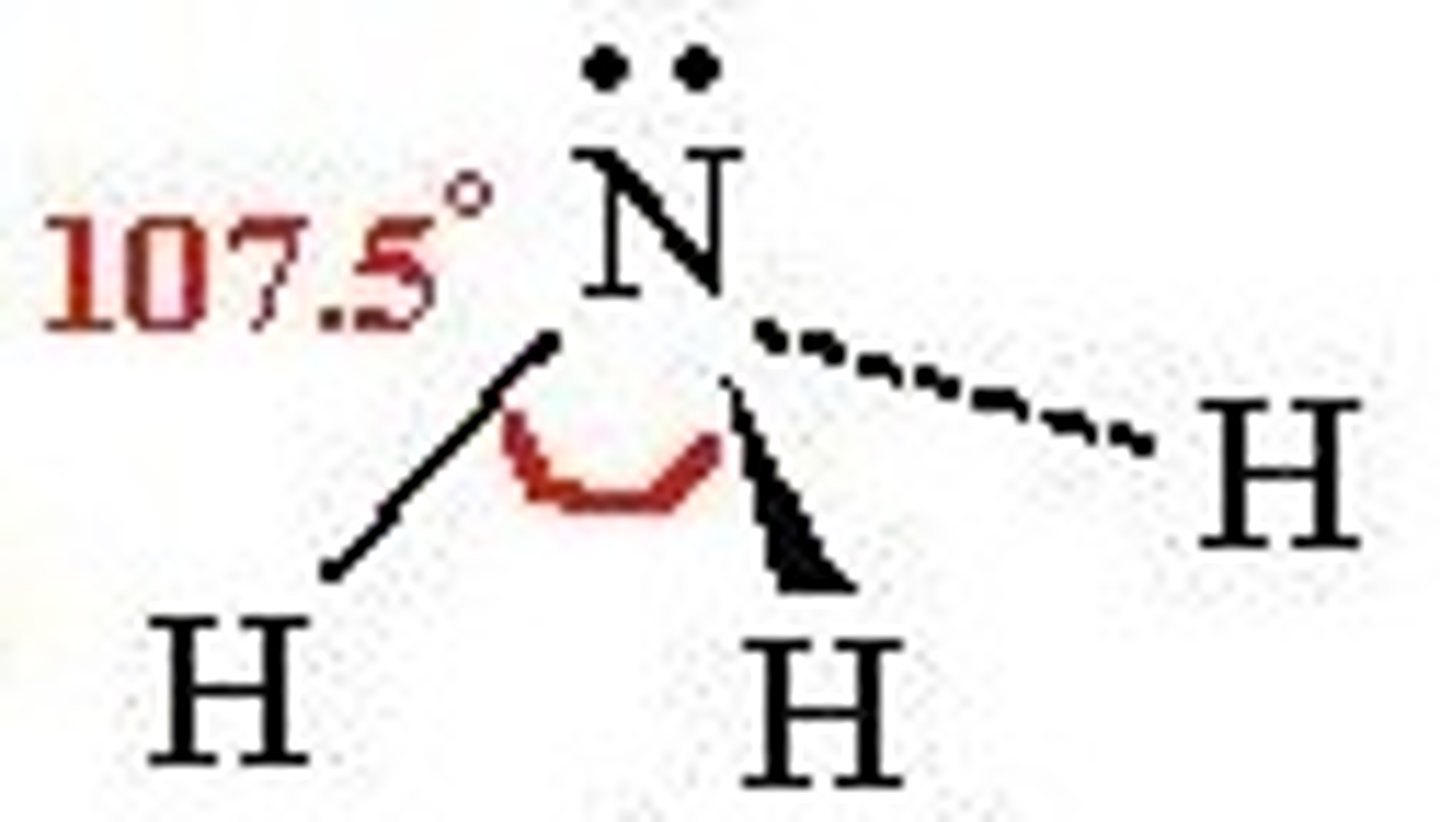
Trigonal Pyramidal
# of Bonding Groups = 4
# of lone pairs = 0
Approximate Bond Angle = <109.5
Example = NH3
6
New cards
Bent (Tetrahedral)
# of Bonding Groups = 2
# of lone pairs = 2
Approximate Bond Angle = <109.5
Example = H2O