chem 107 4/10/2025
1/27
Earn XP
Description and Tags
look on iPad
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Relationship to Other Common Units
The unit used for measuring the oxygen-carrying protein
hemoglobin in the blood is grams/deciLiter, which is the same unit as %
(mass/volume). Hemoglobin levels of 13-18 grams/deciLiter for men and 12-16 grams/deciLiter
for women are considered to be normal.
• To measure molecules such as glucose and cholesterol levels in
the blood, milligrams per deciliter (milligrams/deciLiter) are used. This unit is
also milli grams% (milligram percent). The milli gram in front of the % symbol
indicates that the definition is milli gram per 100 milli Liter. Glucose levels over
110 milli grams% after fasting are considered to be higher than normal
• Total cholesterol levels below 200 milligrams/Liter are considered
desirable.
Parts per Million (ppm) and Parts per Billion (ppb)
Parts per million (ppm) and parts per billion (ppb) are convenient units
to describe very dilute solutions.
• Parts per million (ppm) is sometimes referred to as 1 milli grams/Liter and parts
per billion (ppb) as 1 μgram/Liter.
• Percent mass/volume (% m/v) is parts per hundred. Parts per million
(ppm) and parts per billion (ppb) can be determined by multiplying by a
million or a billion, respectively
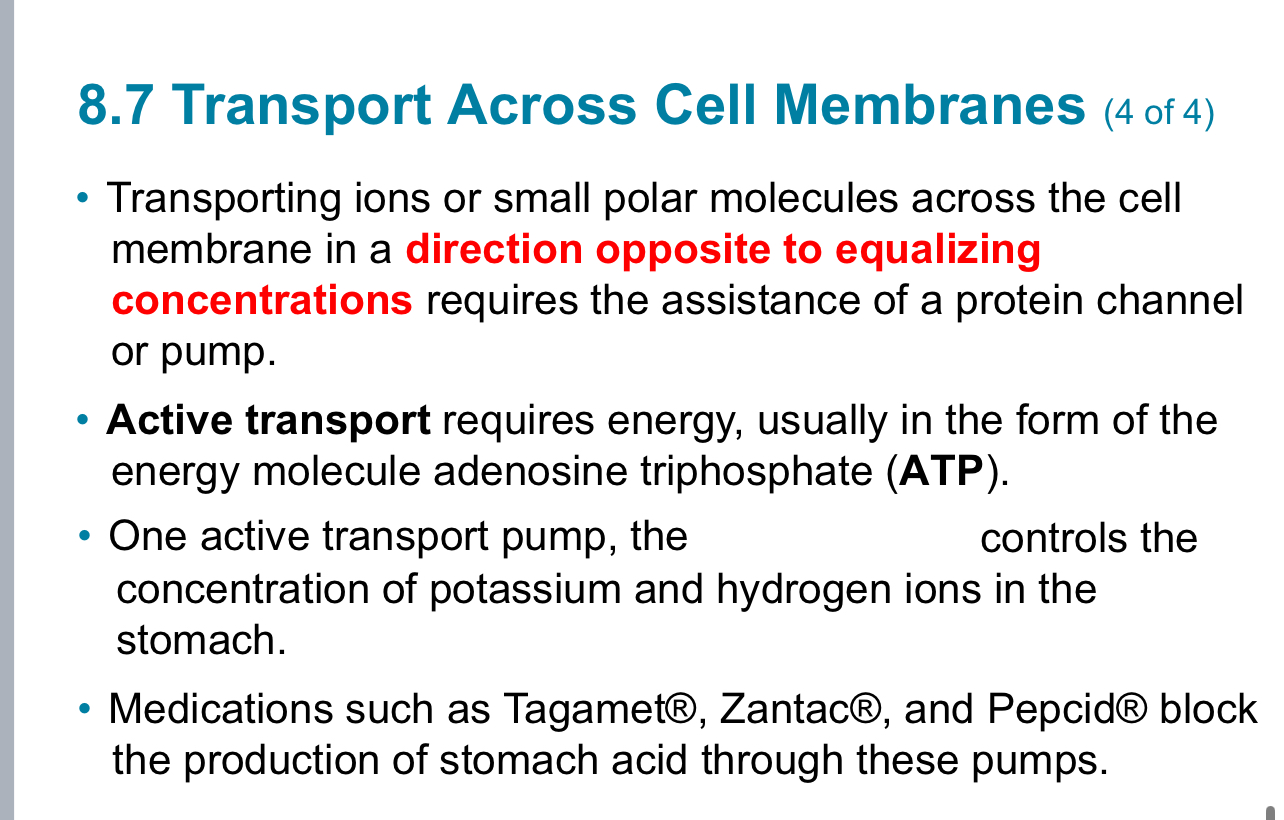
8.5 Dilution (1 of 5)
One way to prepare solutions of
lower concentration is to dilute a
solution of higher concentration by
adding more solvent.
• When you add the concentrated
flavoring in drink drops to water, the
amount of flavoring does not
change even though you have a lot
more solution present – the amount
of flavoring in one squirt originally is
equal to the amount of flavoring in
the water after mixing.
• The amount of solute stayed the
same, but the volume of solution
increased, so the concentration of
the solution decreases.
Dilution (2 of 5)
The following dilution equation represents this
mathematically, where
– Cinitial represents the initial concentration,
– Cfinal represents the final concentration,
– Vinitial represents the initial volume, and
– Vfinal represents the final volume.
• If three of the variables are known, the fourth can be
determined
CinitalVinital=CfinalVfinal
M1V1=M2V2
Dilution (3 of 5)
The dilution equation works with any concentration unit
in which the amount of solution (the denominator) is
expressed in volume units.
• The dilution equation is useful in the health fields because
many pharmaceuticals are prepared as concentrates and
must be diluted.
Using the Dilution Equation
Step 1 Establish the given information.
Step 2 Arrange the dilution equation to solve for the
unknown quantity.
Step 3 Solve for the unknown quantity.
Calculating Molarity After Dilution 1
Calculate the molarity of a solution made by
diluting 0.050 L of 0.10 M HCl solution to a
volume of 1.0 L
• M1 = 0.10 M molarity of solution before dilution
• M2 = Desired Result
• V1 = 0.050 L volume of solution before dilution
• V2 = 1.0 L volume of solution after dilution
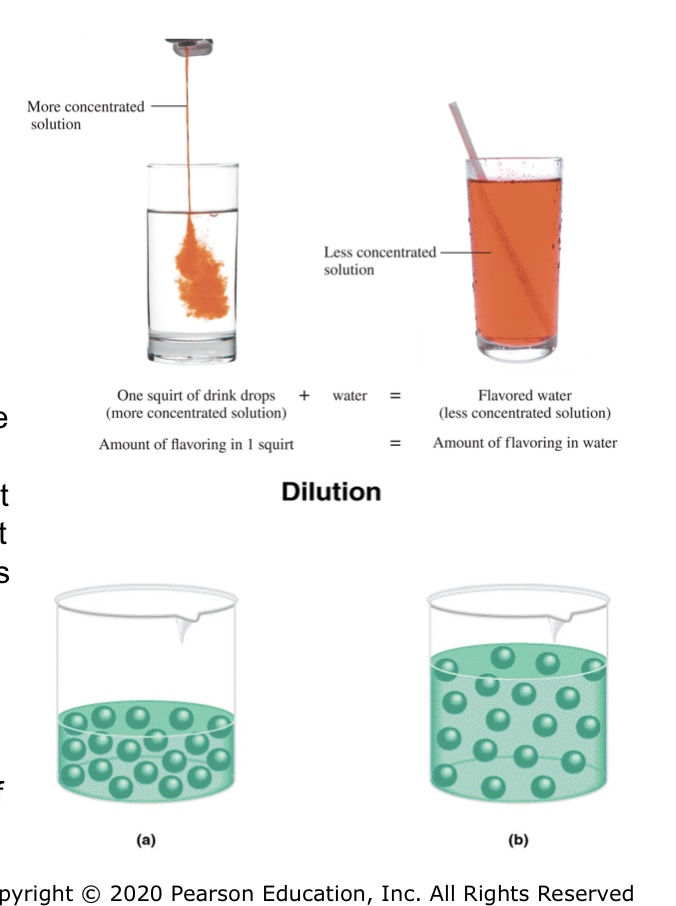
Diffusion
Some types of membranes appear impervious to
Solute molecules cannot cross the membrane as
they are too large
Semipermeable membrane - allows solvent but
not solute to diffuse from one side to anothe
Osmosis
Osmosis - the movement of solvent from a dilute solution to a more concentrated solution through a semipermeable membrane
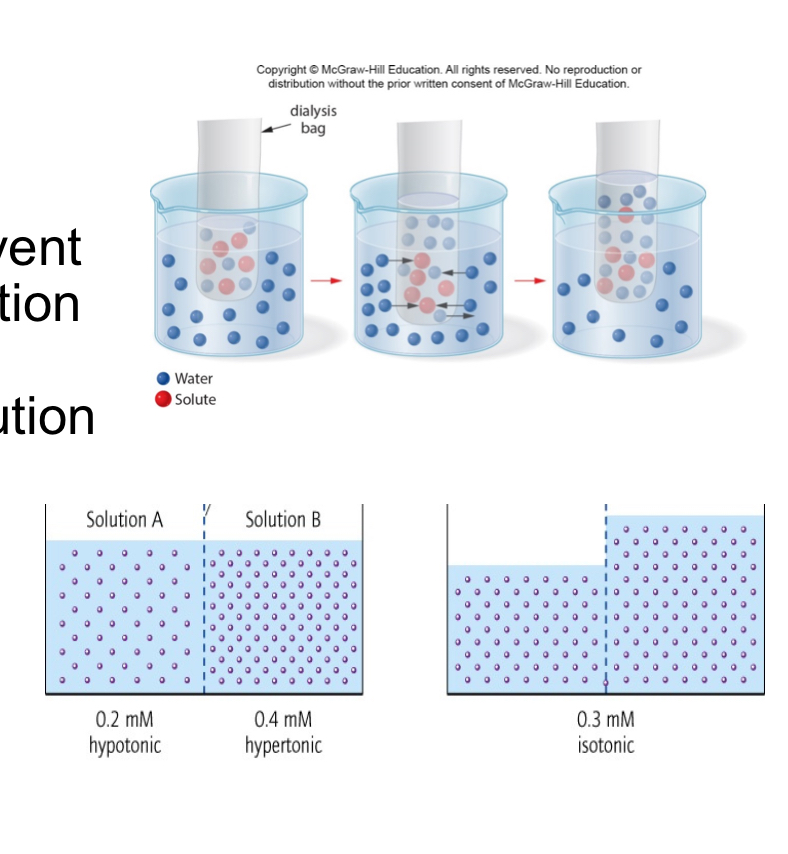
Osmosis
Our bodies are mostly water, composed of a set of specialized
aqueous solutions.
• These solutions are found both inside and outside of cells.
• The concentrations of these solutions is highly controlled by the cells.
• These solutions are separated by a semipermeable cell membrane,
which allows some molecules to pass through but not others.
• A semipermeable membrane allows some molecules to the barrier
but not others.
• Under normal physiological conditions, these are isotonic solutions,
meaning that the concentration of dissolved solutes is the same on
both sides of the membrane
Osmosis
When a person drinks large quantities of water, it dilutes the blood, The
concentration of the solutes in the body’s internal solutions in the blood
goes down, resulting in an imbalance between the concentration of the
solution outside the cells (less dissolved solute) and the concentration
inside the cells (more dissolved solute).
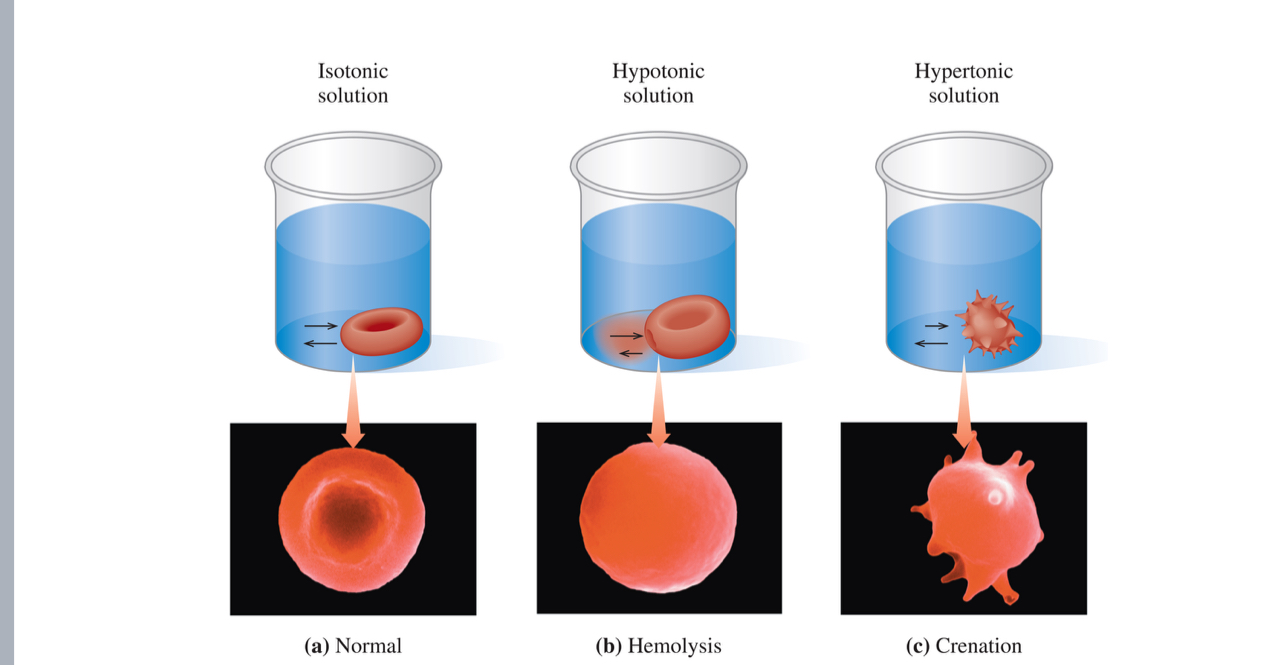
• In this situation, the solution outside of the cells is hypotonic.
• Water will travel across the cell membrane in an attempt to equalize
the concentrations. This passage of water through a semipermeable membrane is called osmosis.
• If too much water enters, the cells swell up and could even burst (a
phenomenon called lysing).
Osmosis
As water flows through a semipermeable membrane during
osmosis, the water molecules in the more concentrated
solution exert pressure on the membrane. This pressure is
termed osmotic pressure.
• The more concentrated the solution, the higher the osmotic
pressure.
• Pure water has an osmotic pressure of zero.
• Applying pressure in opposition to the osmotic pressure will
stop osmosis.
Osmosis
The concentration of dissolved ions in saltwater is about
three times that of the blood.
• When sea water is consumed, it draws water out of the
cells through osmosis, to equalize the concentrations.
• If a person were to drink sea water, the concentration of
solutes in the bloodstream would go up, resulting in a
hypertonic solution.
• During dehydration, the cells shrivel in a process known as
crenation.
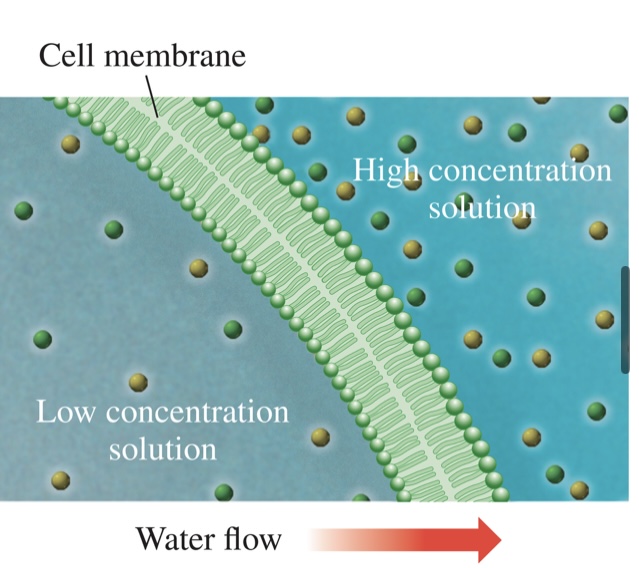
Osmosis and Diffusion (5 of 11)
Osmosis and Diffusion
During osmosis, water flows through
the cell membrane from an area
where the solution is less
concentrated to where it is more
concentrated, to even out the
concentrations.
• The net flow of water will be from the
solution with the lower solute
concentration, diluting the solution
that is more concentrated.
• Water moves from a solution
containing more water molecules
(dilute solution) into a solution
containing fewer water molecules
(more concentrated solution),
equalizing the concentrations
Osmosis and Diffusion
Intravenous (IV) solutions delivered into patients’ bloodstreams are
isotonic. They have solute concentrations equal to the solute
concentrations inside of cells.
• Isotonic solutions minimize osmosis.
• Common isotonic IV solutions used in hospitals include 0.90% (mass/volume)
NaCl (normal saline, NS) and a 5% (mass/volume) D-glucose (dextrose)
solution commonly referred to as D5W (“Dextrose 5% in Water”).
These are called physiological solutions.
• Instead of using a concentration of molarity, physiological
solutions are often express in osmolarity, the molarity times the
number of particles in solution. Osmolarity takes into account the
number of particles exerting osmotic pressure against a membrane.
• Blood plasma has an osmolarity of 0.3 osmoles/L
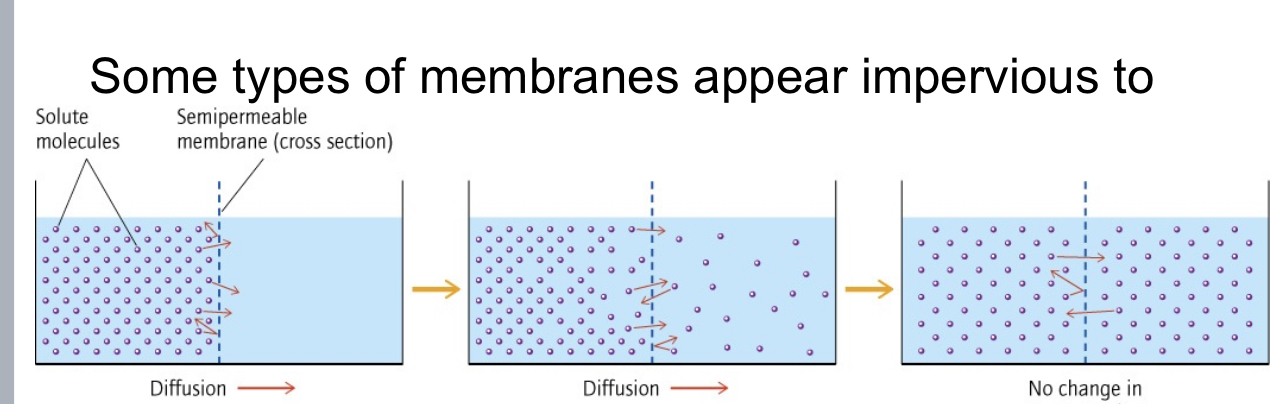
Diffusion and Dialysis
f a drop of green food coloring is put into a
large beaker of water, the green dye
molecules (solute) will mix with the water
(solvent) and the resulting solution will have
a uniform light green tinge to it.
• The two solutions spontaneously mix, and
the green solute molecules diffuse into the
water to form one dilute solution with a final
color intermediate between food coloring
from the dropper bottle and water.
Movement from higher lower concentration!
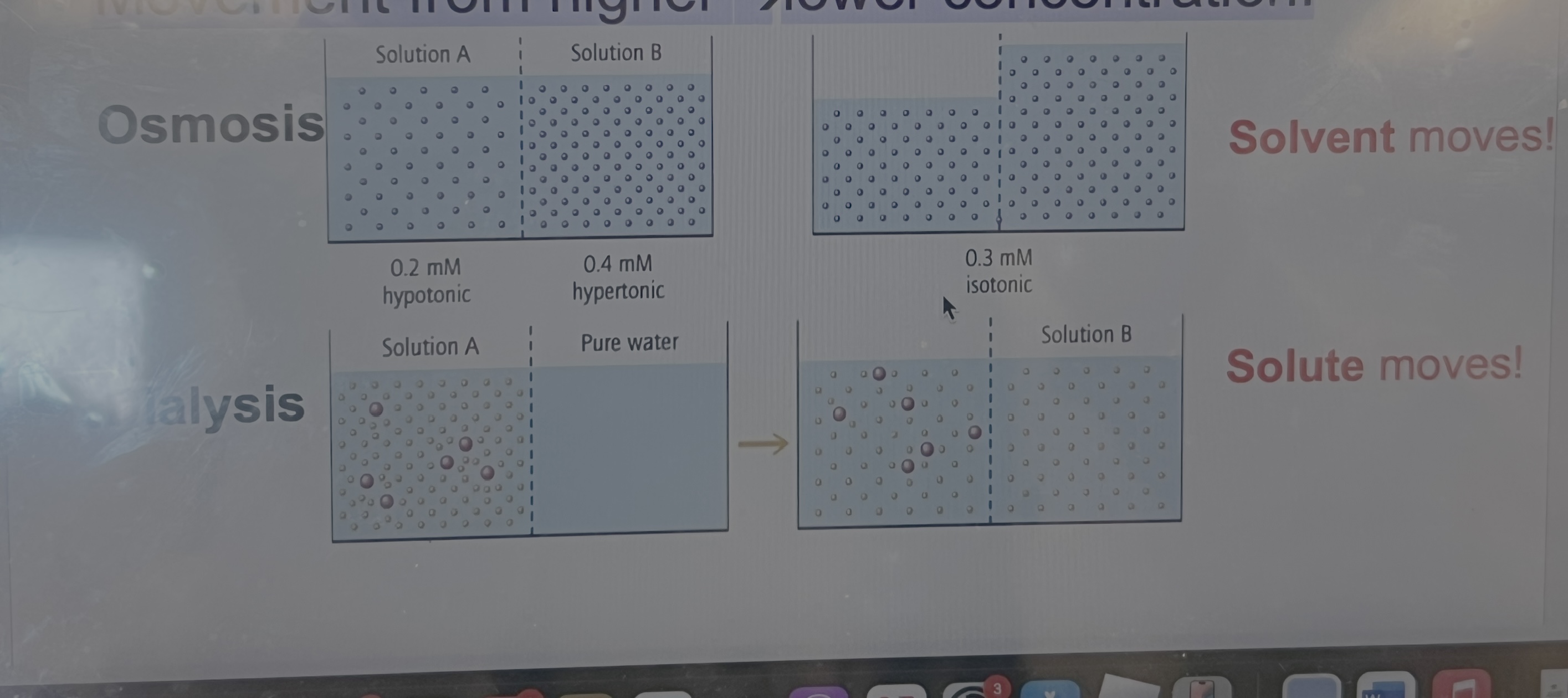
Kidney Dialysis
Diffusion is the movement of molecules in a direction that
equalizes concentration.
• The kidneys act to remove small waste molecules
out of the blood through diffusion across membranes in the
kidneys.
• Cells and larger molecules are reabsorbed into the
bloodstream.
• Small molecules diffuse out of the blood (higher
concentration) and move into urine (lower concentration) in
a process called dialysis.
Osmosis and Diffusion

Transport Across Cell Membranes (1 of 4)
Ions, nonpolar molecules, and polar molecules move
across cell membranes using passive diffusion, facilitated
transport, and active transport.
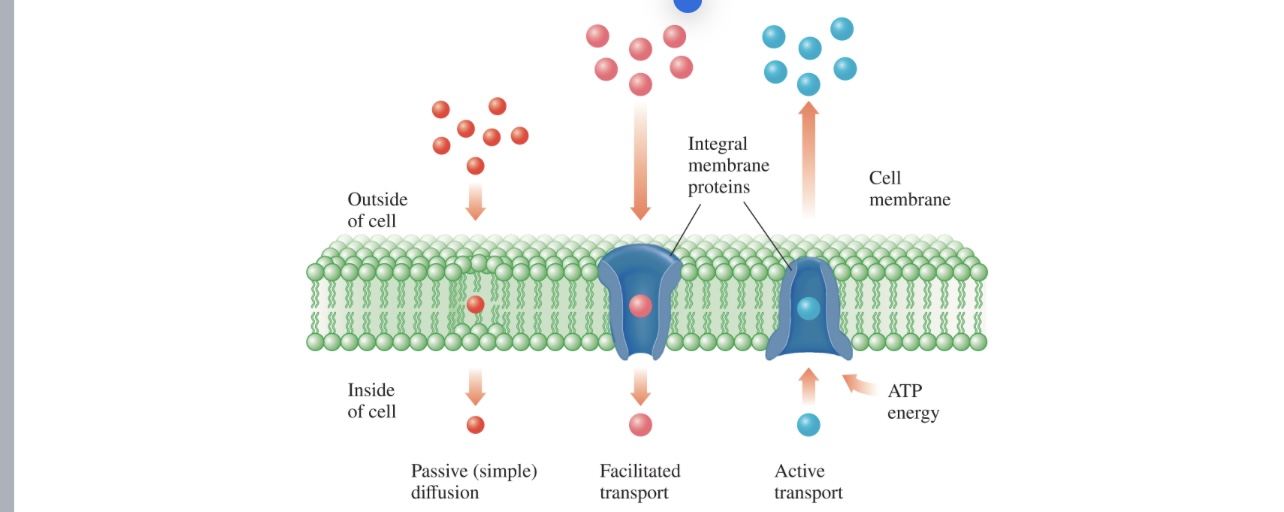
Transport Across Cell Membranes (2 of 4)
Small molecules such as water and the nonpolar molecules
O2, N2, and CO2 can diffuse directly through the cell
membrane.
• Diffusion moves solutes to equalize the concentrations on
either side of a membrane.
• This process does not require any additional energy, so it is
also referred to as passive diffusion.
• Other nonpolar molecules such as steroids can also
passively diffuse through cell membranes
Transport Across Cell Membranes (3 of 4)
To enable small molecules and ions to pass through the
cell membrane, some proteins in the cell membrane have
polar channels that open and close, allowing small
polar molecules and ions to be transported across the
cell membrane.
• These proteins are often integral membrane proteins,
spanning the phospholipid bilayer.
• This facilitated transport does not require energy.
• Glucose transporter proteins are found in virtually all cell
membranes and facilitate transport of glucose into the cell
when blood glucose concentrations are high
Biological Effects of Electrolytes in Solution
The two most important cations in the body
fluids are Na+ and K+

Transport Across Cell Membranes