Equilibrium Law & Equilibrium Constant
0.0(0)
Card Sorting
1/7
Earn XP
Last updated 12:29 PM on 10/28/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
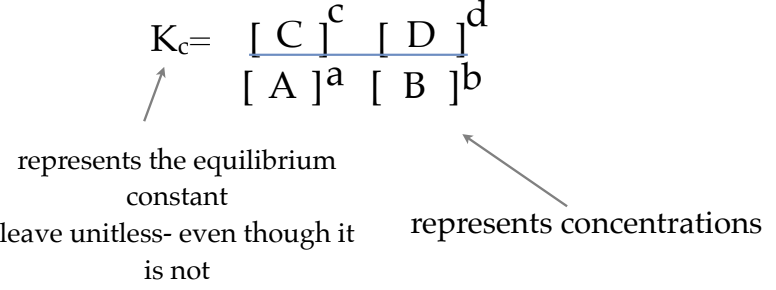
What is the Equilibrium Constant Expression? How is it represented?
the mathematical description of a chemical system at equilibrium using a balanced chemical equation at constant temperature
if we represented an equation as:
aA + bB ⇌ cC + dD
2
New cards
What does Kc represent? What is its unit?
equilibrium constant
no units
3
New cards
What would the Equilibrium Constant Expression of the following equation be?:
cC + dD ⇌ aA + bB
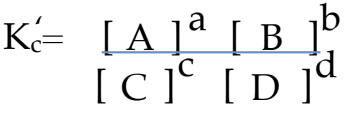
4
New cards
What is the effect on the K value if coefficients are halved?
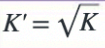
5
New cards
What is the effect on the K value if coefficients are doubled?
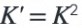
6
New cards
What is the effect on the K value if the equation is reversed?
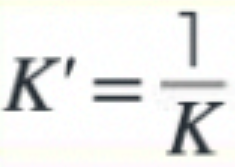
7
New cards
What is the effect on the K value if two equations are added together?
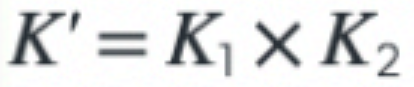
8
New cards
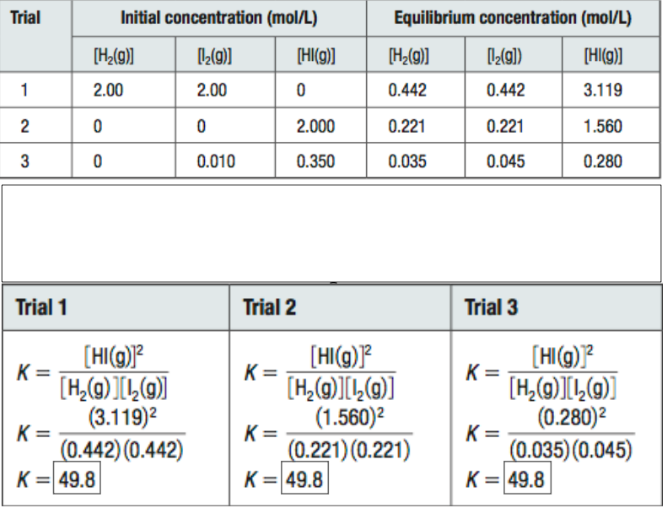
What do these tables tell us about Equilibrium Constants?
Kc ONLY changes with TEMPERATURE
If all other variables remain constant, Kc will remain unchanged for that reaction (equilibrium law)!