Unit 4 - How the Human Body Influences Drug Handling and Action
1/139
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
140 Terms
What is pharmacogenetics?
Pharmacogenetics is the study of an individuals single gene
What is pharmacogenomics?
Pharmacogenomics is the the study of an individuals whole genome
How do we make a functional protein?
There are 23 pairs of chromosomes packed into the nucleus, each is made up of DNA which are sequenced into genes. Each gene encodes for a functional protein. Different gene sequences will give rise to different proteins.
What is a a genome?
An organisms hereditary information, encoded by DNA
What is polymorphism?
Polymorphism is the variation in DNA sequences across a population
What are examples of polymorphism which would affect the efficacy of drugs in the body?
Polymorphism in…
enzymes
receptors
transporters
How does pharmacogenomics improve drug response?
Pharmacogenomics allows for the development of personalised medicines. It allows us to look at the persons gene level and in doing so we can predict whether a medication will be effect/ predict the response the drug will have. This can even be used in infections where we can assess the precise disease-causing infection and deliver the most appropriate treatment
What is expression profiling?
Expression profiling is where we have a number of drugs and we culture the drugs with cells. We then look at the drug which produces the best response - the good responder
What is disease stratification?
Disease stratification is the process whereby we detect the disease expressing gene and then produce a therapeutic agent based on its structure
How would clinical trials have to be modified in order to determine if your pharmacogenetic drug can be used in humans?
Include patients who you know will show a positive response
Exclude/Include patients on their likelihood of having an adverse drug reaction
How do we currently make therapeutic decisions?
Today we give a patient a standard dose and see how they response to the medication
How can metabolic status vary between individuals?
Different people have enhanced or reduced metabolic status
What is the most common CYP enzyme to show genetic variation?
CYP2D6
How does variation in CYP2D6 affect drug delivery?
Genetic variations in CYP2D6 means some people are Poor metabolisers whereby drug can easily accumulate and causes side effects or rapid metabolisers where drug levels are too low so there is a poor therapeutic response
Antidepressant drugs are metabolised by CYP2D6, how would this effect drug dosing in an ultra-rapid metaboliser?
An ultra-rapid metaboliser will show reduced concentrations of drug below the therapeutic window and therefore we would need to take a larger dose of the drug to see a therapeutic effect.
Antidepressant drugs are metabolised by CYP2D6, how would this effect drug dosing in a poor metaboliser?
Poor metabolisers would show an increased concentration of drug and possible drug accumulation as drug is not being eliminated. Therefore, a poor metaboliser may only require 25-30% of the original dose
How is codeine delivery effected by CYP2D6?
CYP2D6 converts the prodrug codeine into the active metabolite morphine and therefore is important for the activation of codeine. In people who are CYP2D6 poor metabolisers, codeine will not be readily converted to morphine and as a result no therapeutic response seen. Whereas, if the person is a rapid metaboliser of CYP2D6 then they will rapidly convert codeine to active metabolite morphine allowing for analgesics effect of codeine. - hence for some people they will say the drug “didnt touch them” but for others it causes sedation and nausea, euphoria for example.
CYP2CP metabolises Warfarin, explain how different metabolic statuses of CYP2CP can effect warfarin dosing.
Ultra-metabolisers of CYP2CP - This would lead to reduced dose of warfarin below therapeutic level and therefore would reduce anticoagulating effect of warfarin meaning blood clots may still occur. INR will be affected.
Slow metabolisers - Reduced metabolism of warfarin could mean levels of warfarin are too high as drug accumulates and is not eliminated and this could lead to increased exposure to warfarin so increased risk of bleeding. INR will be affected.
Comment on how screening of EGFR is required before initiation of gefitinib.
Screening of epidermal growth factor receptor is required in order to know if the anti-cancer drug Gefitinib will be therapeutically active and effective when targeting cancer cells present in the invidual.
Why do we need to know if TPMT is present before treating with azathioprine?
TPMT is an enzyme and prevents the cytotoxic drug azathioprine reaching toxic concentrations in the body. Patient status is required to prevent toxicity
Why is pharmacogenomics not always possible?
Pharmacogenomics is only really used in severe diseases which are chronic
The development of personalised medicines is complex and costly so not routinely used
We would need to upscale clinicians required to perform tests and the cost of equipment to carry out the tests
What family is gentamicin part of?
Gentamicin is part of the aminoglycoside family
Why is gentamicin monitoring a requirement in the UK?
Gentamicin monitoring is a requirement in the UK to ensure concentrations do not enter high/sub-therapeutic dosing and therefore to prevent toxicity
What type of bacteria does gentamicin most commonly treat?
Gram negative bacteria
Why is gentamicin delivered by parenteral administration?
Gentamicin is delivered by parenteral administration because it a highly polar molecule. This means it does not partition through lipid membranes easily and instead favours water. Gentamicin can only partition the membranes by active or facilitated transport. Gentamicin also shows poor oral absorption. The polarity of the molecule can be explained by the number of hydrogen donars and hydrogen acceptors which means that it has high affinity for water. Gentamicin has a LogP of -3.1 showing its poor hydrophobicity.
True or false: Gentamicin is a polar molecule
True
How does gentamicin cause ototoxicity?
Gentamicin can cause ototoxicity which is irreversible damage to the ears. Ototoxicity usually results in bilateral damage. The dose of aminoglycoside/ gentamicin will depend on the risk of ototoxicty, however, there is a genetic susceptibility also. Gentamicin can cause ototoxicity which presents as vestibulea damage causing dizziness or cochlear damage resulting in hearing loss
How does gentamicin cause renal toxicity?
Gentamicin can cause renal toxicity because it can cause acute kidney injury which is typically reversible. Gentamicin causes reduced glomerular filtration rate but does not affect urin output. Renal toxicity is dose dependent.
What factors increase the risk of neurotoxicity in a person taking gentamicin?
Increased risk of nephrotoxicity where:
The patient has hypotension so reduced blood flow to the kidneys
The patient is taking nephrotoxic medications - Contrast media, ACEi, NSAIDs, Diuretics and ARBs
Calculate the dose of gentamicin for a 70kg non-obese patient. Conventional dose is 5-7mg/kg/day in three divided doses every 8 hours
116mg, THREE times a day (350mg/day)
Why may dosing three times a day increase risk of toxicity - conventional dosing regimen.
Dosing gentamicin in three divided doses per day has been associated with an increased risk of ototoxicity and nephrotoxicity and this is because by splitting up the doses we have sustained trough levels which means there is an increased time over which the proxminal convoluted tubule is taking up gentamicin increasing risk of toxicity, as is the case with ototoxicity - sustained trough levels increase exposure time with gentamicin. Whereas, if we were to give once daily doses of THE SAME DOSE we would have high peak concentrations but a reduced time over which gentamicin is taken up by the kidneys and ear, reducing risk of ototoxicity and nephrotoxicity. The kidney PCT would reach a point where its transporters become saturated so toxicity does not really occur
Explain the extended dosing regimen of gentamicin
The extended dosing regimen is where we give gentamicin in one dose instead of three divided doses. This reduces the time in which the body is exposed to gentamicin so reduces the risk of ototoxicity and nephrotoxicity. The extended dosing regimen allows for lower trough levels/ drug free periods in between dosing and this is looked at to know when to give the next dose. Extended dosing regimen results in higher peak concentrations but prevents toxicity as the kidney is saturated by the drug so cannot be uptaken by the PCT, whereas splitting the doses into 3 divided doses would increase the time over which gentamicin is up-taken by the kidney so increases the risk of nephrotoxicity and ototoxicity.
List the advantages of using the extended drug regimen
The advantages of the extended drug regimen…
Reduces the risk of ototoxicity and nephrotoxicity by reducing exposure time
Allows for higher peak levels so increases the bacteria-killing properties of the drug
Allows for reduced trough levels between dosing, these drug free periods between dosing reduce toxicity
Once daily dosing allows for monitoring of bloods to be easier
less nursing time required to administer the antibiotic
When do we take blood samples for monitoring in the extended regimen?
We take blood samples right before the next dose is needed as this allows us to look at trough levels
What are nomograms?
Nomograms are used for extended regimens to determine the time intervals between dosing, this allows us to prevent toxicity, it is a way of ensuring getamicin has been cleared before the next dose is given
True or false: Nomograms are only used for extended regimens
True
What nomogram would we use if we were dosing at 7mg/kg/day?
Hartford nomogram
What nomogram would we use if we were dosing at 5mg/kg/day?
Urban-craig nomogram
True or false: We only use nomograms for drug doses 5mg and 7mg
True
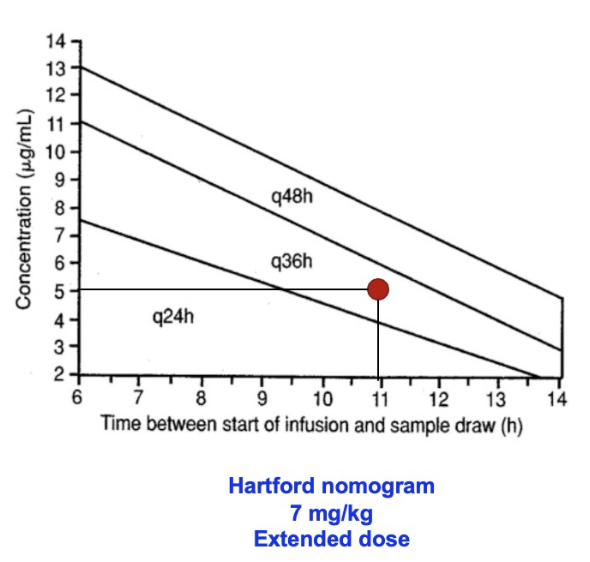
An example: A patient is dosed at 7mg/kg/day and achieves 5mcg/ml at 11 hour post dose, how often should doses be administered?

Every 36 hours
What is the cockgroft-gault formula used for?
A way of determining creatnine-clearance which is an indicator of glomerular filtration used, it is used to determine renal function
Why should we consider renal function before administering gentamicin?
Gentamicin - we should consider renal function because gentamicin is excreted by the kidney and therefore good renal function is important to ensure the drug is adequately excreted
What factors do the cockcroft-gault equation take into consideration?
Age of the patient
Weight of the patient
Gender
Serum creatinine
If we have a malnoruised/ muscle wastage patient, how would this affect creatnine clearance?
Malnourished patient, production of serum creatinine would be low so overestimation of renal function/ high GFR
If we have an overweight patient, how would this affect creatinine clearance?
Obese patients will have lower serum creatinine compared to weight and therefore will have underestimation of renal function and low GFR
If we have a body builder, how would this affect creatnine clearance?
They would have increased serum creatinine and therefore an underestimation of the renal function, low GFR
Why is it so important to consider weight in patients when determining the dose of gentamicin?
It is important to consider weight as if someone has a high weight we need to determine if this is due to fat or muscle. If you have an obese patient, getamicin is polar and therefore does not residue into the fat. Hence using the actual body weight would lead to toxicity in obese patients. Instead we need to use ideal body weight to prevent toxicity in obese patients
Why would we use ideal body weight when determining the dose of gentamicin to administer to patient?
We should consider the ideal body weight of the patient when the patients actual weight is 20% over their ideal body weight. This is because gentamicin is a polar molecule and does not like fat. As a result, by using the patients actual weight it could result in overdosing the patient and toxicity
What creatinine clearance would we consider not using gentamicin?
If Cr/Cl is below 40mL/min reduce dose or consider not using gentamicin/ aminoglycosides
Explain how the dose of gentamicin would be altered in a patient with oedema/ ascities
Does increased due to increased extracellular fluid causing increased volume of distribution
Explain how the dose of gentamicin would be altered in a patient with cystic fibrosis
Increased extracellular fluid so increased volume of distribution
Explain how the dose of gentamicin would be altered in a patient with intensive care patient
increased volume of distribution due to a hypermetabolic state
Explain how the dose of gentamicin would be altered in a patient with a burns patient
A burns victim has changing elimination, initially when we have a burns patient there is hypovolaemia which results in reduced cardiac output and so reduced renal blood flow and liver blood flow. This results in reduced glomerular filtration rate.
We then have a contrary change where we have increased cardiac output, which increases blood flow to the kidney and liver and so increases the glomerular filtration rate.
What drug family does digoxin belong too?
Digoxin is a cardiac glycoside
What is digoxin used for?
Digoxin is used in the treatment of rate/ rhythm control for patients with atrial fibrillation/ atrial flutter
What type of AF, in particular, is digoxin used for?
Long-standing persistent AF/ Non-paraxysmal AF
What is atrial fibrillation?
Atrial fibrillation is a heart condition whereby there is misfiring of electrical impulses from the Sinoatrial node which causes the atria not to properly contract. This misfiring of the SA node also over stimulates the atrioventricular node which means blood is not effectively ejected into the body and the atria to not empty sufficiently for ventricular filling. Insufficient cardiac output as a result
Why are anticoagulants first-line therapy in AF?
DOACS/ Warfarin are important for patients with atrial fibrillation/ flutter as the atria do not contract effectively and as a result you get pooling of blood into the left appendages of the heart, this allows for the blood to lie stagnant and as a result the blood can form blood clots easily - this can lead to pulmonary embolism or stroke.
Why would we not give anti-platelets for AF?
The clot formed in AF is a thrombus clot, it is not platelet-rich and therefore anti-platelets would do very little when preventing blood clot formation.
What drugs do we use (before digoxin) when achieving rate control in AF?
1) Beta blockers - bisoprolol, atenolol, propranolol
2) Rate limiting non-dihydropyridines calcium channel blockers - verapamil, diltiazem
3) digoxin is used only for long-standing persistant AF - ONLY effective at achieving rate control at rest
What patient group is digoxin reserved for?
Sedentary patients
It is no good for people who exercise as it is only effective at achieving rate control at rest.
How does digoxin work, in brief?
Positive ionotropic effect - increases the force of contraction and therefore the rate of contraction of the heart.
What levels of digoxin in the blood would suggest toxicity?
Anything greater than 2.0mcg/L
What side effects do we see in digoxin toxicity?
Side effects in digoxin activity include:
Arrhythmias which can be fatal
GI side effects, especially nausea and vomiting. Diarrhoea can occur.
Dizziness, abdominal pain
When is digoxin monitoring required?
Digoxin monitoring is required when…
Poor compliance is suspected
renal function is declining
hyperthyroidism/ hypothyroidism
drugs that interact with digoxin are co-prescribed
How is digoxin eliminated?
Digoxin is eliminated via the kidneys. It is eliminated, practically unchanged in the urine. Digoxin elimination is reduced in patients with reduced renal function/ reduced via glomerular filtration rate. The half life of digoxin is increased with declining renal function.
How is digoxin distributed?
Digoxin disposition follows a two-compartment model whereby the drug equilibrates with the central compartment and then equilibrates with the cardiac tissue. All other drugs we have seen have been single compartment models.
What are the two routes in which digoxin is delivered?
Digoxin is delivered by both IV and oral route
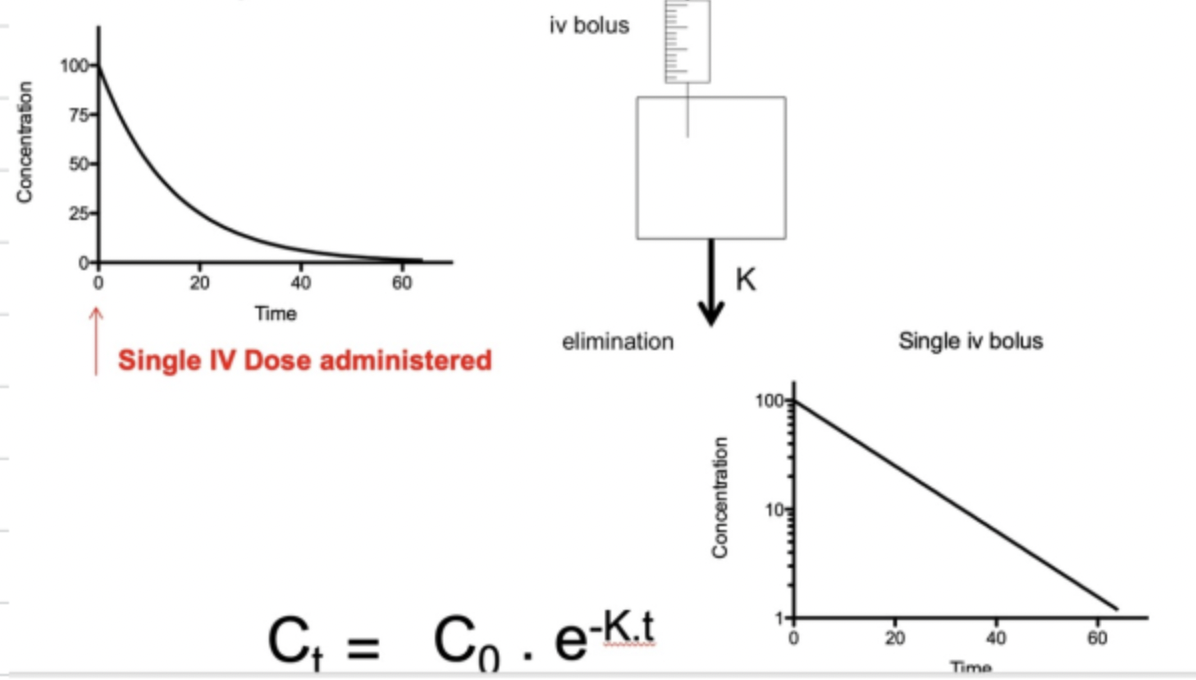
Explain what is meant by a one compartment model

A one compartment model is where we imagine the drug as a box which we directly inject drug into. Once drug is injected, we get instant elimination of drug whereby the concentration of drug in the body declines with time at a proportional rate. This is due to the elimination rate constant
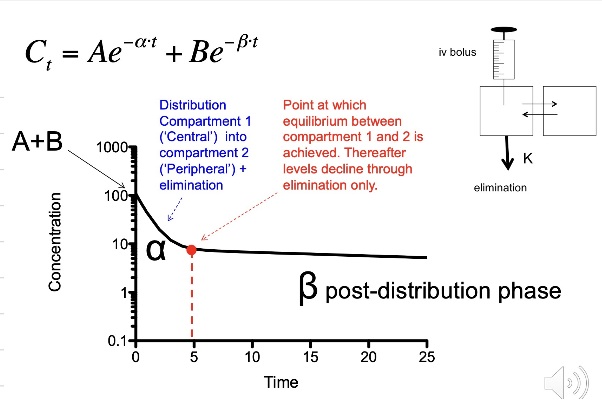

Digoxin is a two-compartment model - explain what is meant by this?
Digoxin follows a two-compartment model: We inject blood into the body, and digoxin equilibrates with the central compartment, e.g., the blood and rapidly equilibrating organs.
We then start to see equilibration with the cardiac tissue, as digoxin has a strong affinity for the cardiac tissue. After one half life, 50% of digoxin is in the cardiac tissue.
After 2 half-lives, more drug moves into the cardiac tissue so concentrations of digoxin increase in the cardiac tissue but decrease in the blood due to distribution and due to elimination.
After 3 half-lives, the drug concentration in the cardiac tissue will continue to increase whilst the concentration in the blood fall due to elimination
After 3-4 half lives, equilibrium between the blood and cardiac tissue is established whereby digoxin reaches steady state. At steady state the concentrations of digoxin in the blood are equal to digoxin levels in the cardiac tissue.
Explain when we would take a blood sample during digoxin monitoring -IV administration
Blood samples are taken after 4-6 hours as it is at this stage steady state is achieved whereby concentrations of digoxin in the blood are equivalent to levels in the cardiac
How would the time in which we take blood samples differ for oral digoxin?
With oral digoxin, we need to account for the time taken for the drug to be absorbed, therefore, we record blood samples after 4-6 hours.
How is the dose of digoxin altered when a patient has hypokalaemia?
Hypokalaemia - If the patient has hypokalaemia then there is increased sensitivity to digoxin by the cardiac tissue and as a result there is increased risk of toxicity. This means that digoxin dose needs to be reduced to prevent toxicity.
How is the dose of digoxin altered when a patient has hypercalcaemia?
If a patient has hypercalcaemia, this means that there is an increased risk of toxicity due to increased digoxin sensitivity in the cardiac tissue. As a result, we need to reduce the dose of digoxin
How would this differ in hyperkalaemia and hypocalcaemia?
Hyperkalaemia and hypocalcaemia would require a dose increase as there is reduced digoxin sensitivity
How is the dose of digoxin altered in a patient who has hyperthyroidism?
Hyperthyroidism - This means digoxin is distributed and eliminated faster and therefore we would require a dose increase
How is the dose of digoxin altered in a patient who has hypothyroidism?
In hypothyroidism, the digoxin is distributed and eliminated a lot more slowly and as a result the dose of digoxin would need to be increased
How does digoxin interact with other drugs? (hint -p-gp)
Digoxin is a substrate of p-gp and this means that it is eliminated by p-gp. Therefore if we have drugs which inhibit p-gp it will reduce the elimination of digoxin and as a result, digoxin levels in the blood will increase which may lead to toxicity. P-gp/ P-glycoprotein is found in the gut and on the kidney and digoxin binds to P-gp causing it to either be effluxed back into the gut to prevent absorption or digoxin is effluxed into the kidney and excreted in the urine. Therefore, drugs that inhibit p-gp will reduce digoxin elimination and as a result may lead to toxicity
What is phenytoin used for?
Phenytoin is an antiepileptic medication - it is used for the treatment of convulsions, specifically tonic-clonic and focal seizures
What is the mechanism of action of phenytoin?
Phenytoin works by inhibiting influx of sodium ions through the sodium voltage gated channels and therefore inhibits the transmission of excitatory neurotransmission
Explain what is seen in terms of protein binding with phenytoin
Phenytoin is mostly bound to proteins in the blood. Phenytoin is about 90% bound to plasma proteins and about 10% unbound in the plasma. It binds to albumin in the blood
How is protein binding affected if a patient has hypoalbuminaemia or reduced renal function?
If renal function is impaired or we have hypoalbuminaemia, then we will see a reduction in the amount of bound drug and an increase in the amount of unbound drug. This is because there are less proteins/ albumin for binding with the drug.
If we increase unbound drug, how does this affect drug concentrations in the blood?
If we increase the amount of unbound drug, we do not necessarily see an increase in drug concentrations in the blood and this is because unbound drug can now undergo distribution into the tissues, reducing blood concentration and can be eliminated which also reduced drug concentrations in the plasma. Therefore, an increase in unbound drug is not necessarily associated with increased drug concentrations in the plasma.
What type of metabolism is seen with phenytoin?
Phenytoin shows capacity-limited metabolism whereby if we increase drug doses, we saturate the enzymes required for metabolism. As a result, we get reduced metabolism so accumulation of drug and increased blood concentrations. This capacity-limited metabolism as a result means therapeutic monitoring is essential as a small increase in drug dosage concentrations can cause a huge increase in blood concentrations and increases the risk of toxicity. When we increase drug concentrations, we saturate the enzymes involved in metabolism, reducing elimination and leading to accumulation of drug. This happens for all drugs but in phenytoin, this can even occur in the therapeutic window.
Capacity-limited metabolism affects elimination. As a result, what other parameters will be affected?
Clearance and t1/2 life
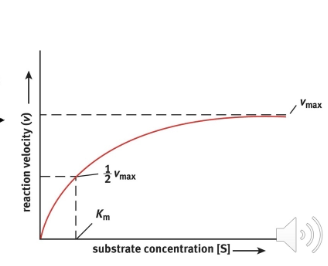
Using the diagram below, define Km and Vmax

Vmax - this is the maximum rate of metabolism of an enzyme system
Km - this is the concentration in which we have half the maximal metabolism rate of an enzyme system
What are the side effects associated with toxicity?
Toxicity is associated with CNS side effects, the side effects displayed with depend on phenytoin concentrations.
Irregular eye movements
Slurred speech
confusion, reduced mental capacity and lethargy
Phenytoin - rate order equations. How do the equations we use differ in phenytoin?
In phenytoin we have have more of a zero order kinetics than a first order kinetics whereby if we have a drug dose of 100mg, plasma concentrations may reach around 5mg/L. We then double the dose to 200mg, concentrations reach 15mg/L. Here we have doubled the dose but tripled plasma concentrations. We then give 250mg, and plasma concentrations increase to 35mg/L, so we have increased plasma concentrations by 15-fold. Hence we do not see first-order kinetics where declining blood concentrations are proportional to elimination. As a result, we cannot use first-order equations as clearance is not proportional, nor is volume of distribution of t1/2
What is first order kinetics?
First-order kinetics is where drug concentrations decline at a proportional rate to elimination
What order of kinetics is seen with phenytoin?
The order of kinetics seen with phenytoin is zero order kinetics
Rearrange the following equation to find the dose of phenytoin: note we cannot use first order kinetics.
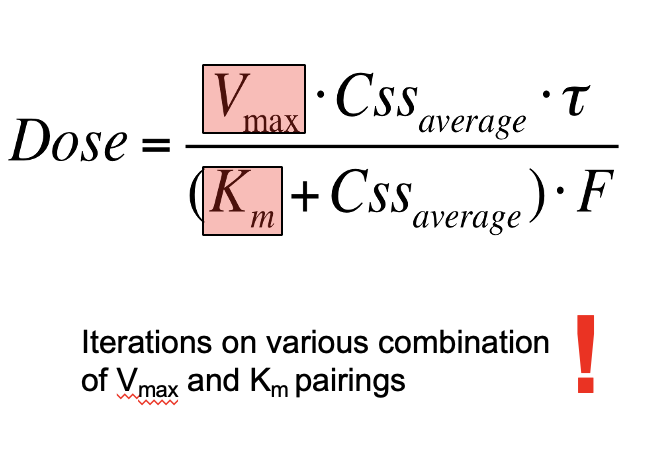
This is used to determine the maintance dose of phenytoin
Note - Vmax and Km will be given
Css average - given
F- oral dose given
T- once daily dosing (1)
What do we use to modify the dose of phenytoin?
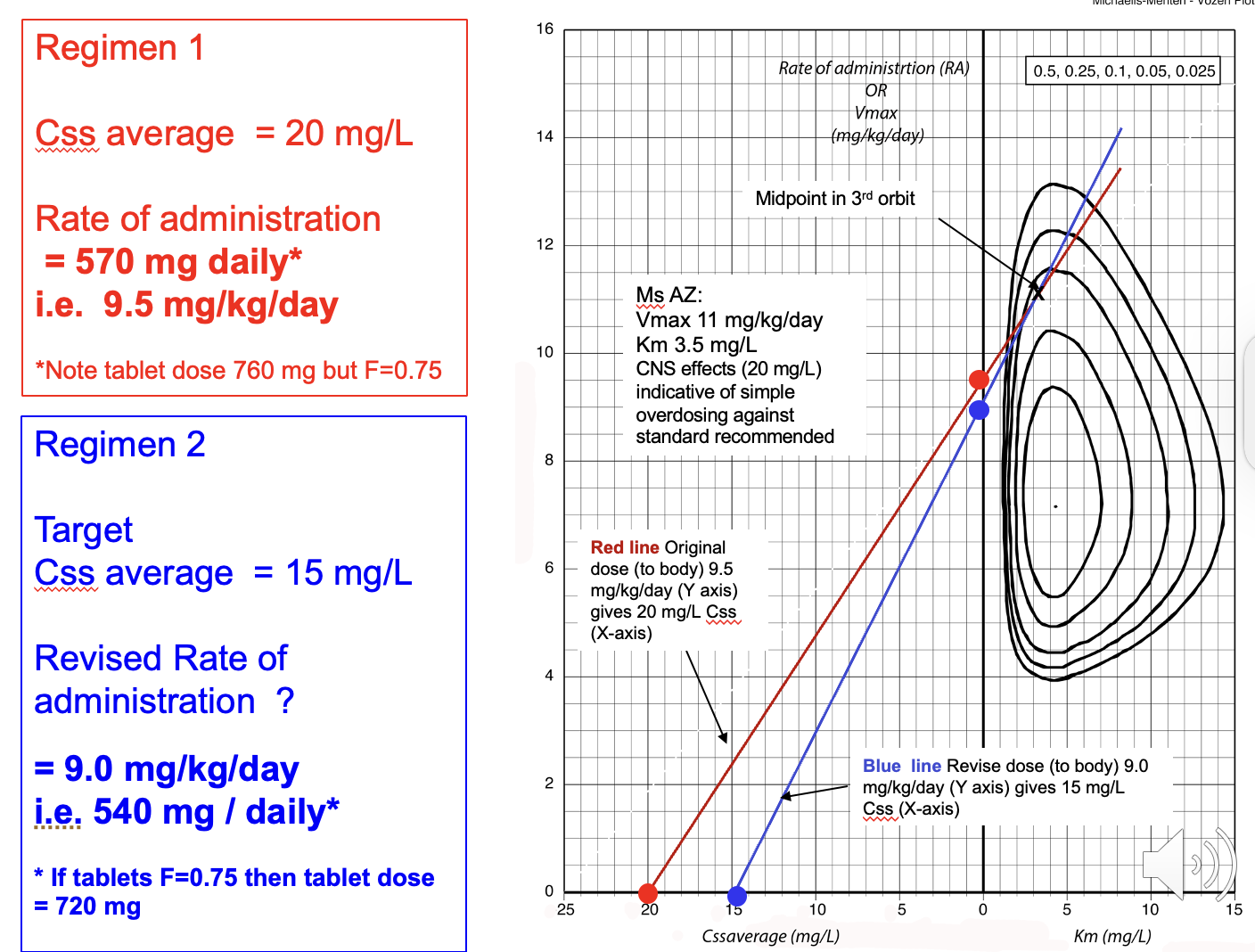
We modify the dose of phenytoin using an orbit plot - this allows us to see the desired dose of drug we need in order to reach a desired steady state concentration
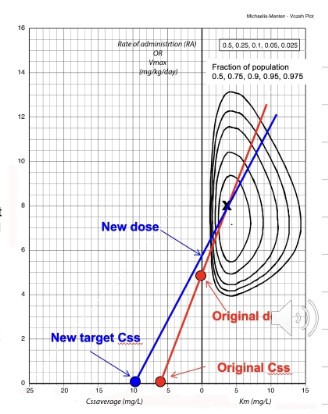
Explain the different section of the orbit plot

Orbit plot - the points at the bottom are the steady state concentrations - provided in question
The line which intercepts the y-axis is the dosage we require. This is calculated mg/kg/day therefore if we know the total daily dose, divide this by the kg to give dose per 1kg.
e.g. 760mg daily dose = 12.6 mg/kg/day (if F=1)
plot that on the orbit plot then at the point of our new desired Css average - intercept the line with the old dose which is intercepting the orbit and this will give the new dose per 1kg so to find total daily dose just multiply it by body weight.
Note that if the bioavailability (F) is 1 - no change is required BUT if F= 0.75 (for example) then multiple the total daily dose at the start by 0.75 and then divide the total daily dose to 0.75 at the end to get tablet dose for F+0.75

Pharmacokinetics - What is therapeutic drug monitoring?
Therapeutic drug monitoring is where we monitor the drug concentrations in the blood in relation to drug dosing. This allows us to monitor drug levels in the blood and the anticipated therapeutic response or adverse effects of the drug concentration.
What is pharmacokinetics?
Pharmacokinetics - Pharmacokinetics is how the body affects the drug. It looks at how following drug delivery, we see changes in drug concentrations: considers PK parameters such as Cl, V, F, and K.
Pharmacodynamics - Pharmacodynamics looks at how the drug affects the body and looks at how drug concentrations change and the therapeutic/adverse effects this causes
Why do we consider both peak and trough levels for therapeutic drug monitoring?
We need to consider both peak and trough levels because…
The peak levels will tell use whether we have reached the therapeutic concentration to e.g. kill bacteria
The trough levels will tell us about sustained drug exposure and in the case of aminoglycosides how this prolonged exposure to drug may increase risk of toxicity
When would we consider the need to therapeutically monitor drugs?
We need to consider therapeutically monitoring drugs when…
There is risk that the drug will cause adverse side effects/ toxicity
Therapeutic drug monitoring is carried out when the drug concentrations in the blood do not necessarily correlate with drug dosing e.g phenytoin which has zero order kinetics due to capacity-limited metabolism
Drugs which are at risk of abuse/ where compliance will likely be effected
Principles of therapeutic drug monitoring: What are the 4 major principles of drug monitoring?
1) We choose a drug regimen that is tailored to the patient, e.g. we need to consider body weight, renal function etc
2) We need to decide at what time intervals we are going to take blood samples
3) We need to monitor the blood samples and compare them with the average population concentrations
4) We then interpret blood samples and change dose if necessary
What sort of parameters does PK take into consideration?
Absorption, Distribution, Metabolism and Excretion
Bioavailability (F), Clearance (Cl), Elimination, volume of distribution (V)
Also takes into account renal function
Css average - sub-therapeutic concentration and toxic concentrations
What bacteria is vancomycin used to treat?
Gram positive (DIFFERS from gentomycin which is gram-negative)