CL2 - Colorectal Cancer
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Principles of surgery in cancer
remove cancer without damaging surrounding structures
lymph nodes and blood vessels supplying the cancer also need to be removed
in bowel cancer the lymph nodes run alongside the arteries so you need to remove them too which removes more bowel than just where the tumour is
Role of surgery
Control local disease
Stages disease (TNM) - radiology cannot tell us this so we need the pathology
Offer cure, extended life (e.g. metastasectomy in CRLM)
Palliate symptoms – stops bleeding, bypass obstruction
Psychological benefits for patients (“just want tumour out”)
Facilitate chemotherapy e.g. reduce malignant cells and thus resistant clones
Risk reducing e.g. BRCA patients
Ways to discuss a disease - “In a surgeons gown, physicians may make some progress”
incidence - how often it happens
age
sex
geography
path - microscopic/macroscopic
symptoms
prognosis
Management of colorectal cancer
Management of any condition in Medicine depends upon diagnosis:
Diagnosis is based upon history, examination and special investigations
Management of cancer follows the above principles at first, and once the diagnosis is made, it all depends upon the stage
Other factors to consider
“Patient factors” (e.g. other comorbidities) and “tumour factors”
“Decisions are more important than incisions”
The art of surgery (and medicine) is in tailoring the treatment to the individual patient (balancing the benefits with the risks/recovery)
Colorectal cancer
CRC (Colorectal carcinoma) - the occurrence of malignant lesions in the mucosa of the colon and rectum.
Colorectal cancers are all adenocarcinomas.
Anal cancer is a different disease
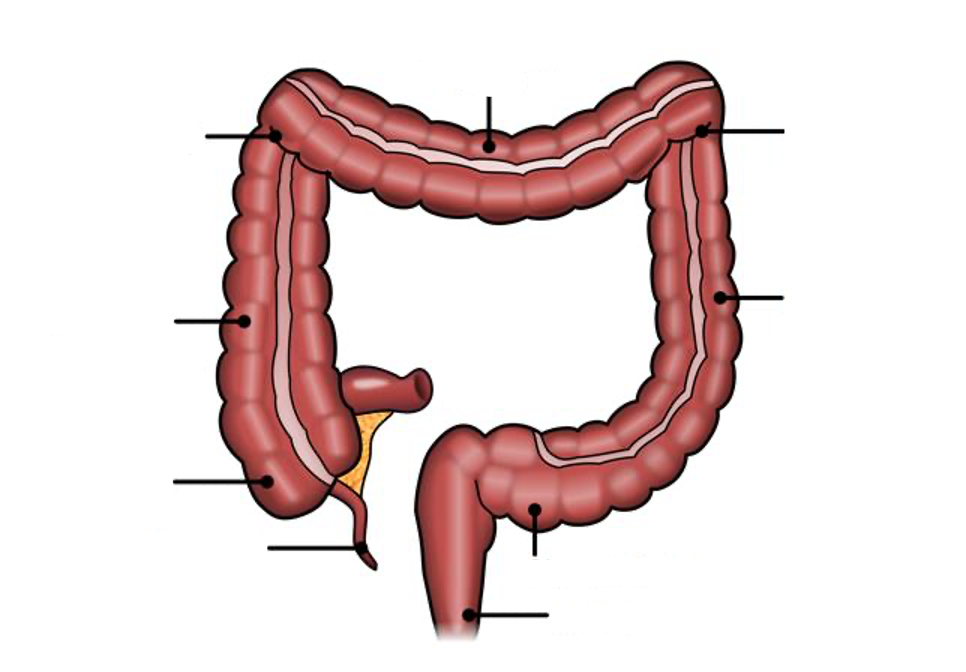
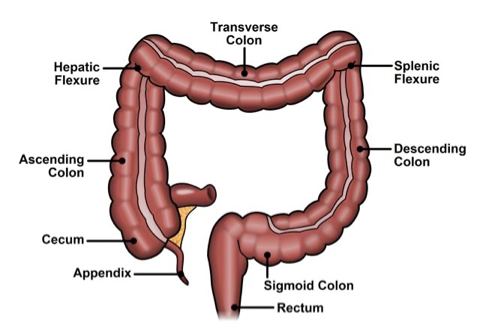
Bowel Anatomy
Bowel Wall Layers
mucosa - epithelium, lamino propria
submucosa - rich in lymphatic and blood vessels (only cancer when it invades this layer through the muscularis mucosa)
muscularis propria - circular and longitudinal muscle
Serosa (visceral peritoneum) and subserosal fat (important in cancer surgery)
Epidemiology of Bowel Cancer
The fourth commonest cancer in the UK (but second commonest cause of cancer death)
1 in 14 men, 1 in 19 women will be diagnosed with CRC in their lifetime
Age – median 60
Sex 1.5:1 M:F
Geography: higher in the West – diet related
Pathology: Macro – Annular, polypoid, ulcerated
Path: Micro – moderately differentiated typical, dirty necrosis
Symptoms of Bowel Cancer
Depends upon location, general ones include:
rectal bleeding
change in bowel habit
weight loss
iron deficiency anaemia
bowel obstruction
Prognosis depends on stage but early detection has around 5 year survival
Pathology of Bowel cancer
Polyps - main cause
Diet – not quite so simple. Interplay with genes.
Family history – 1st degree relatives key
IBD
Genetic Syndromes (HNPCC, FAP) – 75% are sporadic
Previous cancer/radiation
Obesity, smoking, alcohol
Rarer causes:
Ureterosigmoidostomy
Diabetes
Cholecystectomy
Acromegaly
Polyps
a protuberant growth from the mucosa
Can be pendunculated (on a stalk) or sessile (flat)
Pendunclated is better for surgery
Sessile has saline injected underneath it to make it rise slightly above the surface to be surgically removed
Benign epithelial tumour of cells derived from glandular epithelium → mainly adenomas
They are all dysplastic and have disregulated proliferation.
They fail to fully differentiate and are all premalignant (though not all progress to cancer).
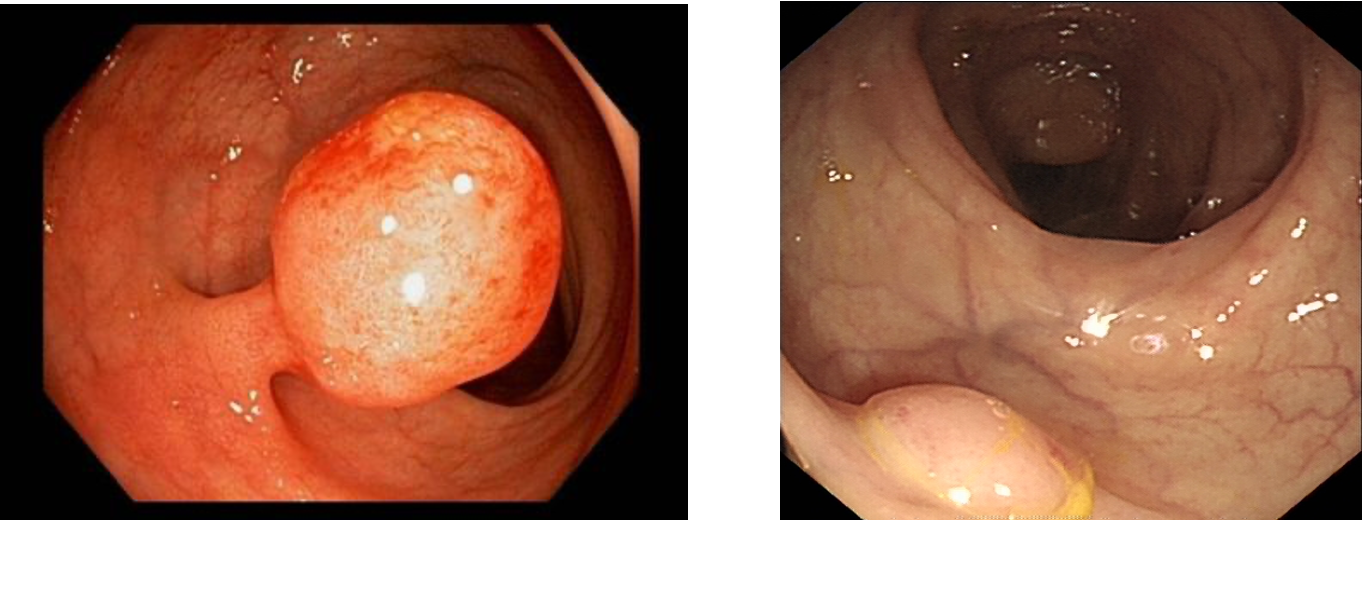
Grading of Polyps
Based on velocity
Tubular adenoma
Villous adenoma
Tubulo-villous adenoma
Non-neoplastic polyps
hamartomas
hyperplastic
Adenoma Crcinoma sequence
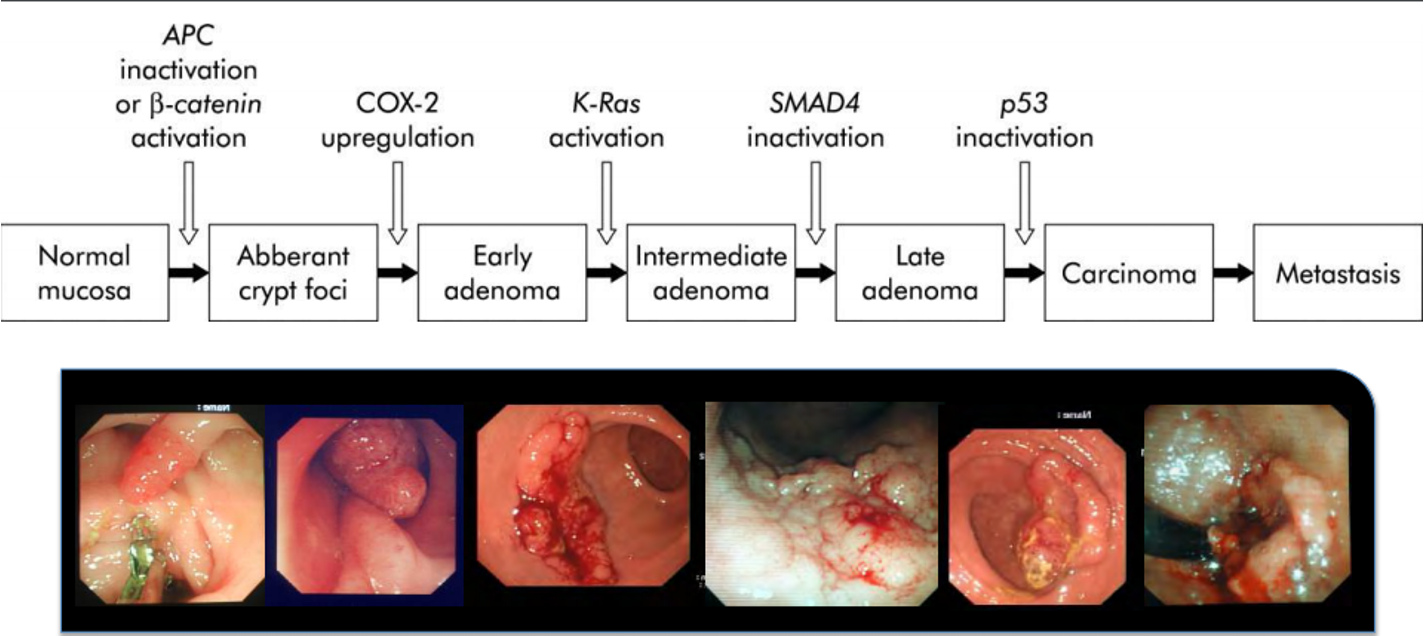
Normal mucosa
→ APC inactivation or B-catenin activation ~ Wnt pathway
Causes abberant crypt foci
→ COX-2 upregulation
Causes early adenoma
→ K-Ras activation
causes intermediate adenomas
→ SMAD4 inactivation
causes late adenoma
→ p53 inactivation
causes carcinoma which progresses to metastasis
Evidence for adenoma-carcinoma sequence
Distribution of polyps matches cancer and background polyps in cancer
Carcinomas found in adenomas (“polyp-cancer”)
Coexistence in high risk groups e.g. FAP
Follow-up of patients declining polypectomy
Pathology of Bowel cancers
98% are large bowel/colon cancers are adenocarcinoma.
Other rare tumours depend upon the cell of origin that has become malignant (NET, lymphoma etc)
Macroscopic classifications
Annular (circumferential – causes bowel obstruction)
Polypoidal - can cause anaemia
Ulcerated (2/3) - can perforate
Grading of Bowel cancer
How much like normal tissue does it look like?
Grade I – well differentiated (15%)
Grade II – moderately differentiated (70%)
Grade III – poorly differentiated (15%)
Modes of spreading
Direct –invades other structures through the bowel wall e.g. bladder, abdominal wall
Lymphatic – critical. Basis of original Dukes staging
run with the blood vessels and are a critical aspect of surgery
Haematogenous – metastases get to liver via portal vein
25% of CRC patients present with mets
Transcoelomic – spread throughout the peritoneal cavity.
T4 cancer spreads and sheds of cancer cells in abdominal cavity
Classically to ovaries – Krukenberg tumours (MASSIVE tomours in the abdominal cavity) – very chemoresistant
Implantation – suture line, wound, laparoscopic ports sites
Dukes Pathological staging of bowel cancer
Stopped being used in 2018 and was replaced with TNM
Dukes A: Confined to bowel wall
Dukes B: Through bowel wall
Dukes C: Lymph nodes involved
Dukes D: Distant Metastases
Genetic syndromes
Two key inherited bowel cancer syndromes are HNPCC and FAP
Associated with other cancers
Screening of patient and family
HNPCC
Hereditary non-polyposis colon cancer - Also known as Lynch syndrome – slight distinction between them in terms of MMR status
Inherited colon cancer
5% of new cases per year
Germline mutation in Mismatch repair gene (MMR) which is a tumour suppressor gene which corrects wrong base pairing
Have microsatellite instability – DNA repeats
Average age of diagnosis 45
Usually develop on the right
Synchronous and metachronous
Biologically aggressive, rapid transformation from benign to malignant
Associated with other cancers – must screen for them (though these are not as aggressive as de novo of the same):
Endometrial cancer
Ovarian cancer
Gastric
Amsterdam Criteria
identifies high risk families for genetic testing (3,2,1)

FAP
Familial adenomatous polyposis
1% of all CRC
APC mutation on 5q – β-catenin and Wnt pathways
100% risk of CRC by 20-30s
Autosomal dominant inheritance
Multiple extra-intestinal manifestations
Originally defined by the presence of >100 colorectal adenomas
Difference between family history bowel cancer
General rule of thumb is:
relative right, life left
General Symptoms
Anorexia
Weight loss
Anaemia
Fatigue - severe
Right sided - Local Symptoms
Abdominal mass
Iron deficiency anaemia (IDA)
Small bowel obstruction
Perforation
Symptomless
Left sided - Local Symptoms
Rectal bleeding
Change in bowel habit
Bowel obstruction/abdominal colic
Mucus discharge (more often associated with a large polyp)
Fistula – to e.g. bladder
Perforation
25-30% of patients with left sided colon tumours present as an emergency – usually LBO/perforation
Rectal Tumours
Rectal bleeding (60% of patients)
CIBH including mucus PR
Tenesmus (sensation of incomplete evacuation even if you went - brain can’t distinguish the difference between stool and the lump)
Rarely fistula
Signs of bowel cancer
Get this from the rectal exam
Conjunctival pallor from anaemia
Cachexia - pt looks very skinny and washed out
Abdominal Mass
Palpable rectal mass
Palpable liver/jaundice in sclera (eye)
Lymphadenopathy
Rectal examination is critical - can assess lesion size/nature (i.e. hard cancer or soft polyp), location in relation to sphincters, local fixity/mobility, sphincter quality
Synchronous vs Metachronous
Synchronous - more than 1 cancer at the same time
Metachronous - more than 1 cancer in 10 years
Distant Disease
Also affects:
Liver – jaundice, RUQ pain, ascites
Lung – incidental on scan often, SOB
Other – lymphadenopathy, bony pain
Investigating Bowel Cancer
Barium enema – outdated now – traditionally used
due to lower sensitivity of detection of CRC
(82-85%)
Endoscopy: flexible sigmoidoscopy and colonoscopy – latter is Gold standard
CT Colonoscopy – Good sensitivity/specificity. Cannot biopsy. Good for completion “scope” if obstructing tumour/cannot complete endoscopically
Sigmoidoscopy is the same camera as colonoscopy but stops earlier
Loco-regional staging
CT C/A/P – looks for mets.
Occasionally used in elderly, frail unfit patients used to rule out large obvious lesion cause they wont tolerate a colonoscopy
MRI for rectal cancer good for CRM and “N” stage
TRUS for early rectal cancer – assess suitability for local excision – good for “T” stage only really
Endoscopy
Flexible sigmoidoscopy and colonoscopy – same scope but end points different
Flexible fibre optic tubes
better for patients who can’t tolerate the colonoscopy such as IBD patients
Bowel prep +/- conscious sedation
90% caecal intubation (we are audited on this)
1:1000 perforations
Can biopsy, tattoo and treat
CTC/CT pneumocolon
Less invasive
Pretty much 100% caecal imaging
High sensitivity for polyps and cancer
Can assess other intra-abdominal structures
CEA blood test
Carcinoembryonic antigen
good at baseline - if tumour is a CEA secreting lesion it can be a good test for surveillance and recurrence
Management of this Cancer
principles of cancer resection
what are you trying to achieve - reduce local recurrence, cure, palliation, prolonging life
if non-metastatic remove the mets to stop a local problem and staging
lymphadectomy key - for staging and treatment
Management: If the cancer is Metastatic disease
Secondary or primary dealt with first?
deal with mets first if fatal unless primary is causing anaemia and more damaging etc
Types of Bowel Cancer Surgery
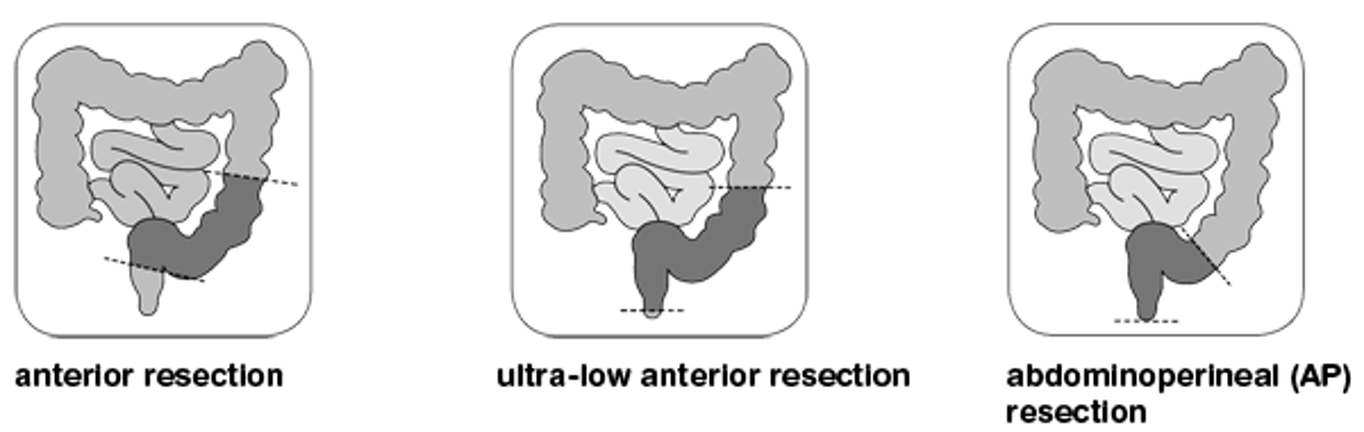
Methods for colon removal surgery
Can do open, laparoscopic (“keyhole”), robotic or transanal if rectal cancer
Risks of this include:
Anaesthesia
Bleeding
Infection (chest, wound, urine)
Anastomotic leak
Injury to other structures (e.g. ureter)
Stoma
MI
DVT/PE
Death
What are the steps post surgical removal of cancer?
Once the specimen is out and pt has recovered
Await histopathological assessment – this is key to prognosticate and decide further treatment
Defines the stage of tumour: TNM and excision margins
If no blood vessel or nodal involvement the patient is considered surgically cured
Continue surveillance to ensure doesn’t develop new primary or metastatic disease
Decisions after the surgery
If nodal or vascular invasion is present– consider giving “adjuvant” chemotherapy to reduce the risk of developing metastases
If margin is involved this is bad news…. May need to consider further treatment such as radiation to the tumour bed, not that effective…
What can surgery help with?
Local recurrence
Overall survival/Disease free survival (governed by metastases)
In-hospital mortality
Quality of life (nerves of sexual/urinary and bowel function)
Surgery will cure cases whereby the cancer can be resected with an R0 resection
Principles of CRC surgery
Resection of not only primary tumour but draining lymphatics and blood vessels within mesenteric envelope
Complete tumour removal (R0)
Dissection in precise embryological, “holy” planes to produce an undisrupted mesocolic/mesorectal specimen
holy plane means resecting the primary cencer and the package of tissue it is housed in
Ontogenetics
The mapping of body compartments established during early embryologic development
Traditional cancer surgery is based on wide excision with a safe margin
The ontogenetic theory of local tumour spread claims that local dissemination is facilitated in the ontogenetic compartment of origin, but suppressed at its borders in the early stages of cancer development.
Optimal local control of cancer is achieved by whole compartment resection with intact margins following ontogenetic “planes”.
MRI role in cancer
MRI tells us which cases of rectal cancer stay in this ontological envelope
Designed to reduce risk of developing metastases
Best given to “Dukes C” patients
Usually 5FU based: 3-6 months
Work on DNA
Problems related to toxicity
Examples of chemotherapy drugs
5-Fluorouracil – interferes with RNA synthesis and DNA replication
Leucovorin – potentiates 5FU
Oxaliplatin – cross links DNA – thus inhibits synthesis
Irinotecan – Topoisomerase inhibitor
Bevacizumab – VEGF inhibitor
Cetuximab – EGFR inhibitor – only for Ras WT
Screening/Prevention of bowel cancer
Bowel cancer screening programme – biannual – 60-74
Faecal occult blood testing:
If negative – repeat in 2 years
If positive – offered colonoscopy:
At scope - 50% normal, 40% adenoma, 10% cancer
Bowel scope is new – flexi sig for all >50yrs
Surveillance/Prevention of Bowel Cancer
Family History
Genetic syndromes: Lynch, FAP – prophylactic surgery
Previous cancer
Previous polyp
Standard Follow-up post CRC surgery
CT C/A/P years 1 and 2
Colonoscopy years 1 and 5 (some do year 3 also)
CEA 6 monthly for first three years, then annually for 2 more
Generally stop after 5 years – considered cured