Chemistry Exam - Year 10 🙏😭🥀
1/46
Earn XP
Description and Tags
{EMOJI UPDATES} Chemical Patterns, Chemical Reactions, Organic Chemistry
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
Metal element + oxygen = ? 🙏
Metal Oxide (Combustion)
Hydrocarbon + excess oxygen = ? 😭
Carbon Dioxide + Water (Combustion)
AB ➡ A + B 🔨
Decomposition
A + B ➡ AB 🍜
Combination
Active metal + acid ➡ ? 🧬
Salt + Hydrogen
Metal carbonate + acid ➡ ? 🎸
Salt + Water + Carbon dioxide
Hydrochloric Acid ➡ ? 🤨
HCl
Nitric Acid ➡ 🫨
HNO3
Sulfuric Acid ➡ 😴
H2SO4
Test for Carbon dioxide…?
Put a lighted splint into a sample of the gas
Test for Hydrogen…?
Put a lighted splint into a sample of the gas
Test for Oxygen…?
Put a glowing splint into a sample of the gas
What is a Precipitate…?
A solid that forms as the result of a chemical reaction in aqueous solution
What is Metal Displacement…?
When a more reactive metal displaces a less reactive metal from its salt solution or compound
What is this…? (126C, 136C, 146C)
Isotopes
Why when you go down the periodic table does the Atomic radius increases…?
Each new period adds a new electron shell
What data can we extract from 1319 K?
The number of protons, neutrons, and electrons in an atom.
What is the electron configuration of 3919 K?
2,8,8,1
What period of the periodic table is 3919 K?
Potassium is in Period 4
How reactive is 3919 K?
It is very reactive
What is the charge and cation/anion of 3919 K
1+ Cation
What is Metallic…?
Cations surrounded by a sea of delocalised electrons
Name properties of Metallic Bonding
High Melting Point, Ductile, Malleable, Conductor
What is Metallic bonding…?
Bonding between metals
What is Ionic…?
A direct electron transfer occurring from a metal to a non metal.
What is ionic bonding…?
Bonding between ions
What is Covalent bonding…?
Bonding between Non-Metals
What does Covelant mean…?
When non-metal atoms share electrons to achieve a full valence shell
What is the cheat trick for writing ionic formula? 🥳🥹
Swap the charges between the two atoms
Properties of ionic substances…?
High Melting Point, Brittle, Hard
Properties of Covalent substances…?
Low Melting Point, Poor conductors, Can exist in all stages
Flame Test experiment learnings are…? 🔥
Heating a salt solution, a flame colour is visible, electrons from ground state are promoted to the excited state then releases a photon in the form of light when dropping down to the ground state.
What separates the crude oil…? 🛢
Fractional distillation tower
What is volumetric analysis…? 🧪
Determine the concentration of a solution by measuring the volume of a second solution that reacts with it.
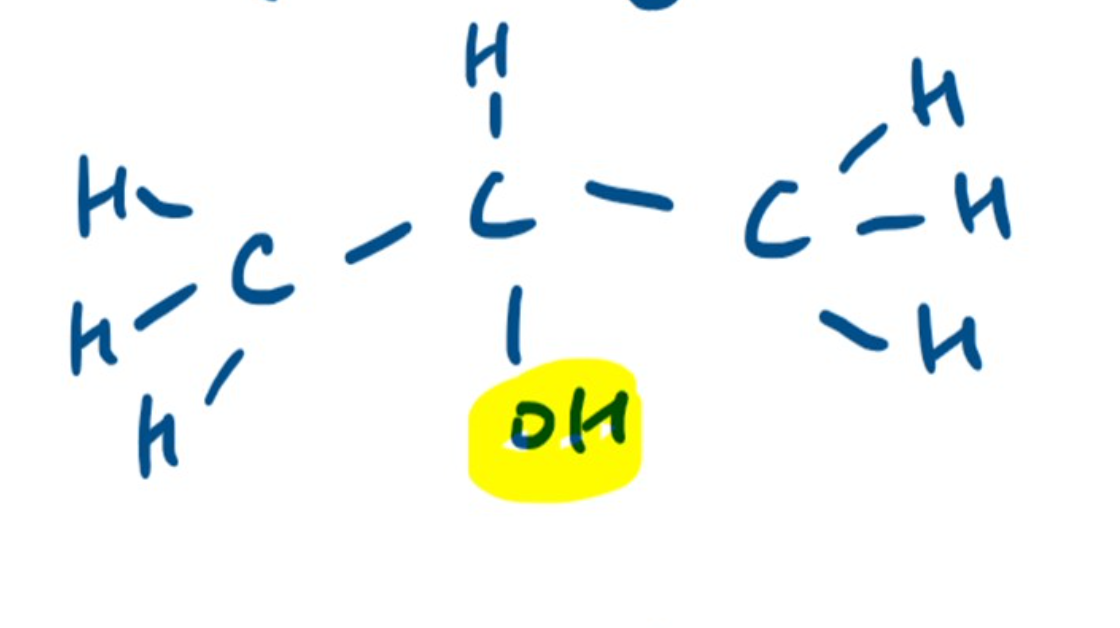
Name the Alcohol 🍻
Propan-2-ol
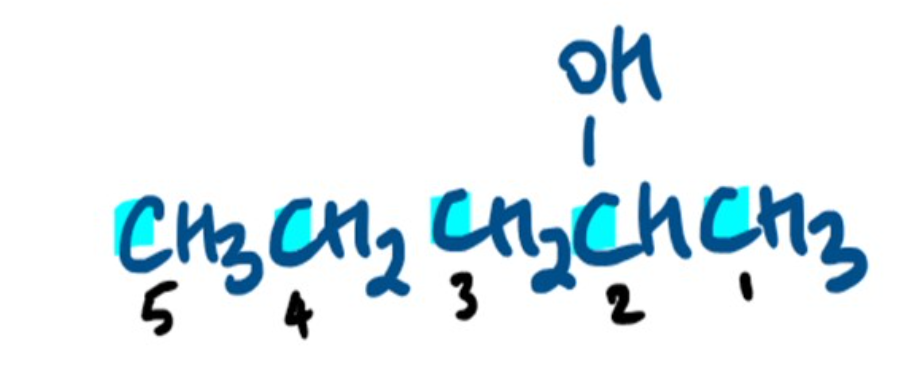
Name the Alcohol 🍻
Pentan-2-ol
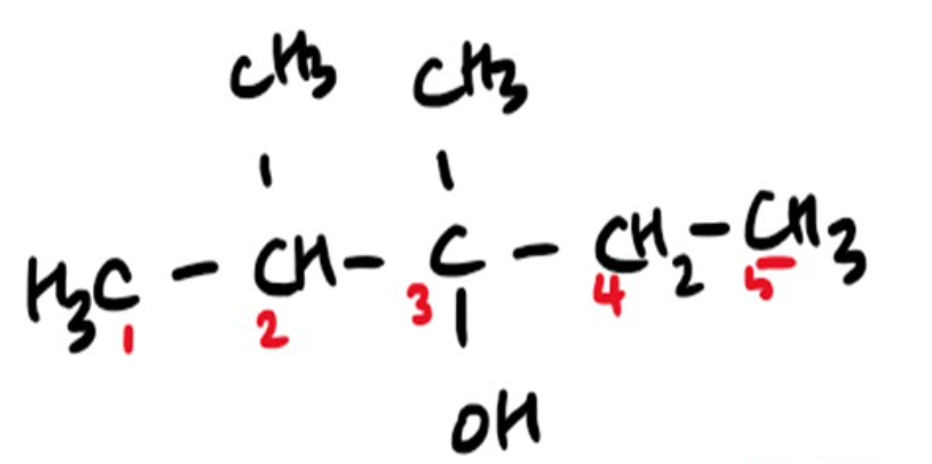
Name the Alcohol 🍻
2,3 dimethyl pentane-3-ol
What is an “Alcohol”…? 🍺
Contains an OH group
What does an Alcohol make an Alkane do…? 🏆➡💧
Not soluble in water to soluble
How many Carbon is attached to the Central carbon in a Primary? 📕
1 Carbon
How many Carbon is attached to the Central carbon in a Secondary? 📗
2 Carbon
How many Carbon is attached to the Central carbon in a Tertiary? 📘
3 Carbon
Why is OH part of a functional group…? 💨
Because it is an atom when added to a hydrocarbon changes its chemical/physical properties
What is an isomer…? 🫣
Same formula but a different arrangement of atoms in the molecule and different properties.
What number is a “mole” or better known as Avogradros constant? 🥑
6.02×1023
What is a solution…? 👨🍳
Solvent + Solute when uniformly mixed
Can light pass through a solution…? ⚡️
Yes