Synthetic polymers
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
How is an addition polymer formed?
Many small molecules called monomers join together to create very large molecules called polymers
What are the rules to drawing the repeat unit of an addition polymer?
No brackets
Double bond into single bonds
Single bonds should extend to the sides
Everything else stays the same
Draw the repeating unit of poly(ethene)
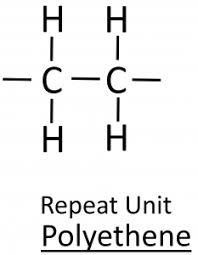
Draw the repeating unit of poly(propene)
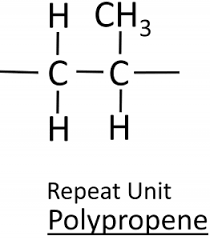
Draw the repeating unit of poly(chloroethene)
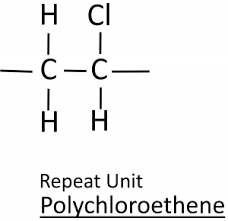
Draw the repeating unit of poly(tetrafluroethene)
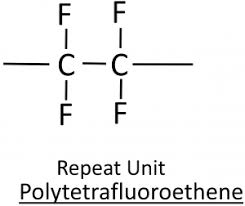
What are the problems in disposal of addition polymers
Their inertness (inability to react), means that microorganisms and bacteria are unable to break them down, causing accumulation
When they are burned, carbon dioxide is released which contributes to global warming
What is condensation polymerisation?
The process which monomers combine to form a polymer, while releasing small by-products like water
What does the term diol mean?
It means the presence of 2 alcohols
What is the general formula for making a polyester?
diol + dicarboxylic acid —> polyester + water
How does condensation polymerisation work for polyesters to form?
A diol (alcohol with 2 OH groups) bonds with a dicarboxylic aicd (carboxylic acid with 2 COOH groups)
As part of the process, the dicarboxylic acid loses the OH group off of each COOH group
the diol loses the H off of each OH group
Remaining molecules join together to make a polyester
H and O join to form water
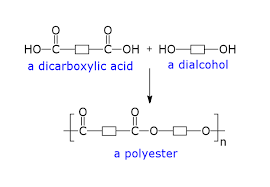
What are the rules to drawing the repeating unit of a polyester?
Identify the OH from carboxylic acids and H from alcohols to form H2O
Draw just one of each diol and dicarboxylic acid and a single bond at each end
No brackets
What is the structural formula for a reaction between ethanedioic and ethanediol
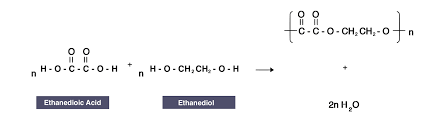
Which type of polyesters are biodegradable?
biopolyesters, meaning they are easier to dispose of
can be made from lactic acid obtained from cornstarch