A1 - Molecules
1/24
Earn XP
Description and Tags
(A1.1) Water and (A1.2) Nucleic Acids
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Where did the first cells originate?
In “primeval soup”, an aqueous solution of water with other substances dissolved in it. Oceans basically.
List the reasons (8) why water is a substance on which life depends:
Metabolism: Chemical reactions.
Nutrition: Reactions of photosynthesis and digestion.
Growth: Cytoplasm is aqueous, cells must absorb water by osmosis to increase in size.
Reproduction.
Movement.
Response to stimuli: Nerve impulses, hormones.
Excretion: Urine, waste gases requires a moist surface.
Homeostasis: Blood plasma and tissue fluid.
Describe the structure of an atom and the nature of ions.
Smallest unit of matter. Consists of protons (+), neutrons (N/A) and electrons (-). A deficit or surplus of electrons makes an atom an ion. A cation has a positive charge and results from the loss of electrons. On the other hand, an anion has a negative charge and results from the gain of electrons.
Outline the formation of ionic and covalent (polar and nonpolar) bonds between atoms.
Ionic bonds form when one atom transfers electrons to another, resulting in an attraction between a positive and negative ion.
Covalent bonds form when two atoms share a pair of electrons.
Polar: Sharing is unequal.
Nonpolar: Sharing is equal.
Draw water molecules (including notation of charges).
(See image)
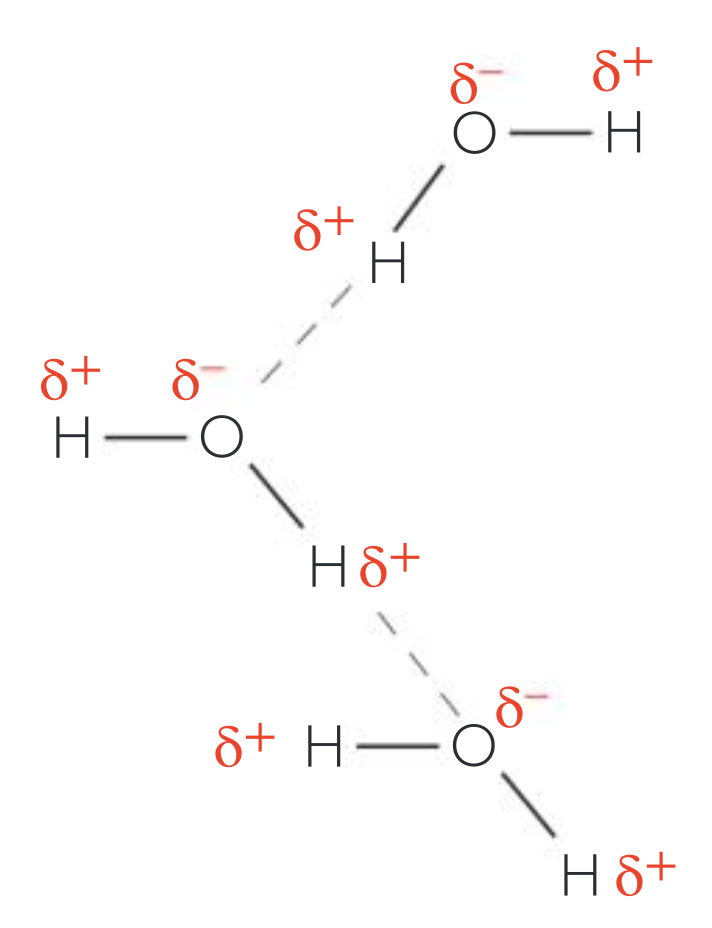
Where is the polar covalent bond located within a water molecule?
Between each H and O.
Explain the partial charges.
Water molecules are polar because electrons are more attracted to the O nucleus, which makes it slightly negative while the H atoms are slightly positive.
Why do Hydrogen bonds form between water molecules? Discuss their strength.
These intramolecular forces form between the positive pole of one molecule and the negative pole of another. An individual bond is weak but each water molecule can form up to 4 hydrogen bonds with other water molecules. This collective effect makes water strong enough to have its properties (i.e., high boiling point, surface tension, etc.)
What are two examples of how organisms use this property? Explain the mechanisms of each.
Conduction of water in xylem: As transpiration occurs in plants, it creates negative pressure (suction) which pulls water upwards. Because of cohesion, the columns of water molecules do not break and are instead a continuous stream.
Water surfaces as habitats (e.g. water striders, mosquitoes): Even though some organisms are denser than water, surface tension allows water to be a cohesive structure that resists breakage.
Why is water attracted to polar/charged molecules?
Water itself is polar. So, polar or charged molecules are hydrophilic because of electrostatic attractions between them.
Outline the cause of capillary action. Describe it in plant tissue.
Due to adhesion of water molecules to the surfaces of the spaces. In plants, water adheres to cellulose. When water evaporates, adhesion pulls water from the nearest xylem vessel, keeping the walls moist.
Describe the role of capillary action in soil.
Soil is made of particles with pores between them. Water moves through these pores upwards. This is how water can rise from underground and how roots have access to moisture.
Discuss water’s properties as a solvent. Use the terms “polar/charged”, “hydrophilic” and “hydrophobic”.
Because water is polar, the partial charges in its individual atoms allow it to surround and separate other polar or charged (hydrophilic) molecules, forming “shells” around the solutes. On the other hand, nonpolar molecules are hydrophobic so they instead group together.
State an example of the function of a molecule depending on it being hydrophobic and another depending on being hydrophilic.
Glucose is polar, dissolves in blood plasma, and can be easily transported to cells for energy.
Phospholipids have hydrophobic tails and hydrophilic heads which allows them to form cell membranes.
Outline the role of water as a medium for metabolism.
Water dissolves reactants and enzymes, allowing them to collide and interact. It also stabilizes temperature and is sometimes even used to break down bonds (hydrolysis).
Outline the role of water as a medium for transport in vascular plants and in animal blood.
Plants: Water transports minerals from the roots and sugars form the leaves via xylem and phloem.
Animals: Water is the main component of plasma which transports nutrients (amino acids, glucose), hormones, waste products (urea) and gases.
List and explain (4) physical properties of water that are consequential for animals in aquatic habitats.
Buoyancy: Upward force exerted by water to support the weight of objects. Solids float in fluids if their density is lower. Helps organisms spend less energy.
Viscosity: Water’s high resistance to flow due to cohesion. Makes movement harder so animals have streamlined bodies to reduce drag.
Thermal conductivity: Ability of a material to transfer heat. Helps aquatic animals maintain stable body temperatures and keeps environment temps even.
Specific heat capacity: Quantity of heat needed to raise the temperature of a gram by one degree. Water’s high heat capacity means that it can keep aquatic environments thermally stable and it also protects organisms from sudden temperature fluctuations.
Compare the physical properties of water to those of air.
(see image)
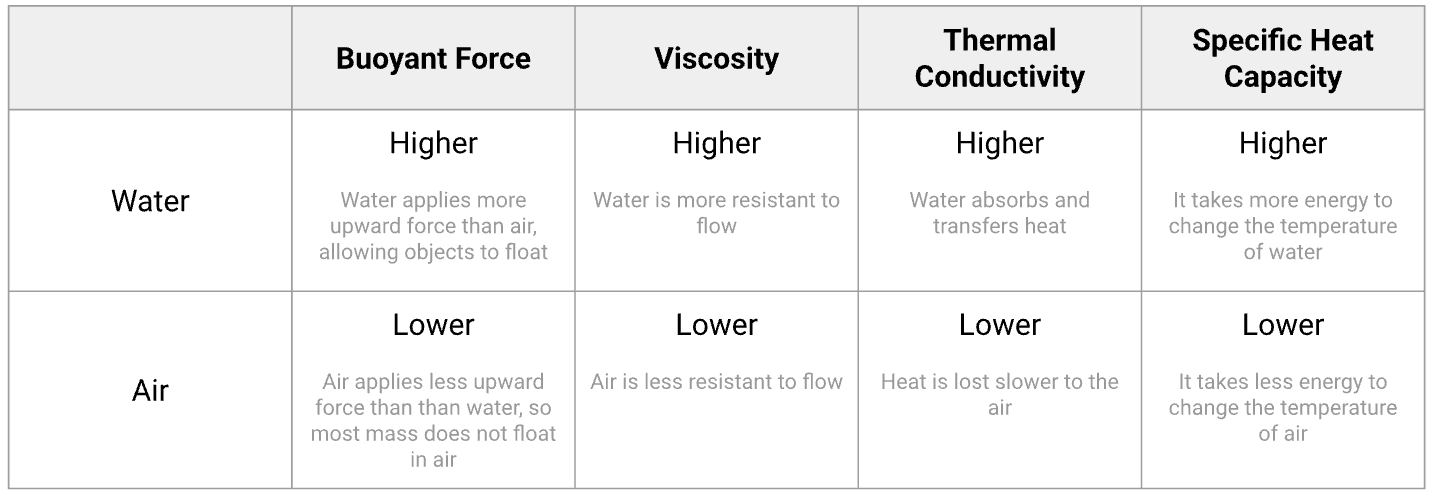
Describe how the black-throated loon (Gavia artica) and the ringed seal (Pusa hispida) interact with the physical properties of water in their habitat.
Buoyancy in water allows the seal to stay afloat without expending a lot of energy. However, the water is viscous, so the seal has adaptations for streamlining as it swims through it. Water has a greater thermal conductivity than air, so the seal needs to insulate itself with blubber to maintain body temperatures. However, because the water has a high specific heat, the temperature of the water does not change as rapidly as the air around it, providing habitat stability for the seal.
Buoyancy in water allows the loon to stay afloat without expending a lot of energy, however when flying through air the loon must expend energy to stay aloft. Air is not viscous, so the loon can easily move through it when flying. The loon doesn't lose as much body heat to the air because air has low thermal conductivity. However, because the air has a lowspecific heat, its temperature changes as rapidly.
Explain the leading hypothesis of the origin of water on Earth.
Asteroids containing ice collided with the early Earth. The ice would have melted, adding to the volume of liquid water in the growing oceans.
State two reasons why water was retained on early Earth.
Strong gravitational pull due to Earth’s size.
Intensity of sunlight due to distance from the Sun which keeps the Earth below 100°C and mostly above 0°C.
Explain why the presence of water is considered fundamental to the search for extraterrestrial life and the “Goldilocks zone”.
Water is a unifying feature in biology so it’s reasonably expected for life to be found in planets with liquid water. To retain liquid water, a planet must be large enough for gravity to be strong and temperatures must be warm enough for ice to melt, but not so hot that water boils. This means that planets must be in the “Goldilocks zone” which is the range of distances from a star that keep temperatures between 0-100°C.
Adhesion
Water molecules sticking to another hydrophilic substance.
Cohesion
Water molecules sticking together because of hydrogen bonding.
Solvation
Interaction of a solvent with the dissolved solute.