3.3 Quantum Numbers & 3.4 Electron Configurations & 3.5 Drawing Molecular Orbitals
0.0(0)
0.0(0)
Card Sorting
1/11
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
1
New cards
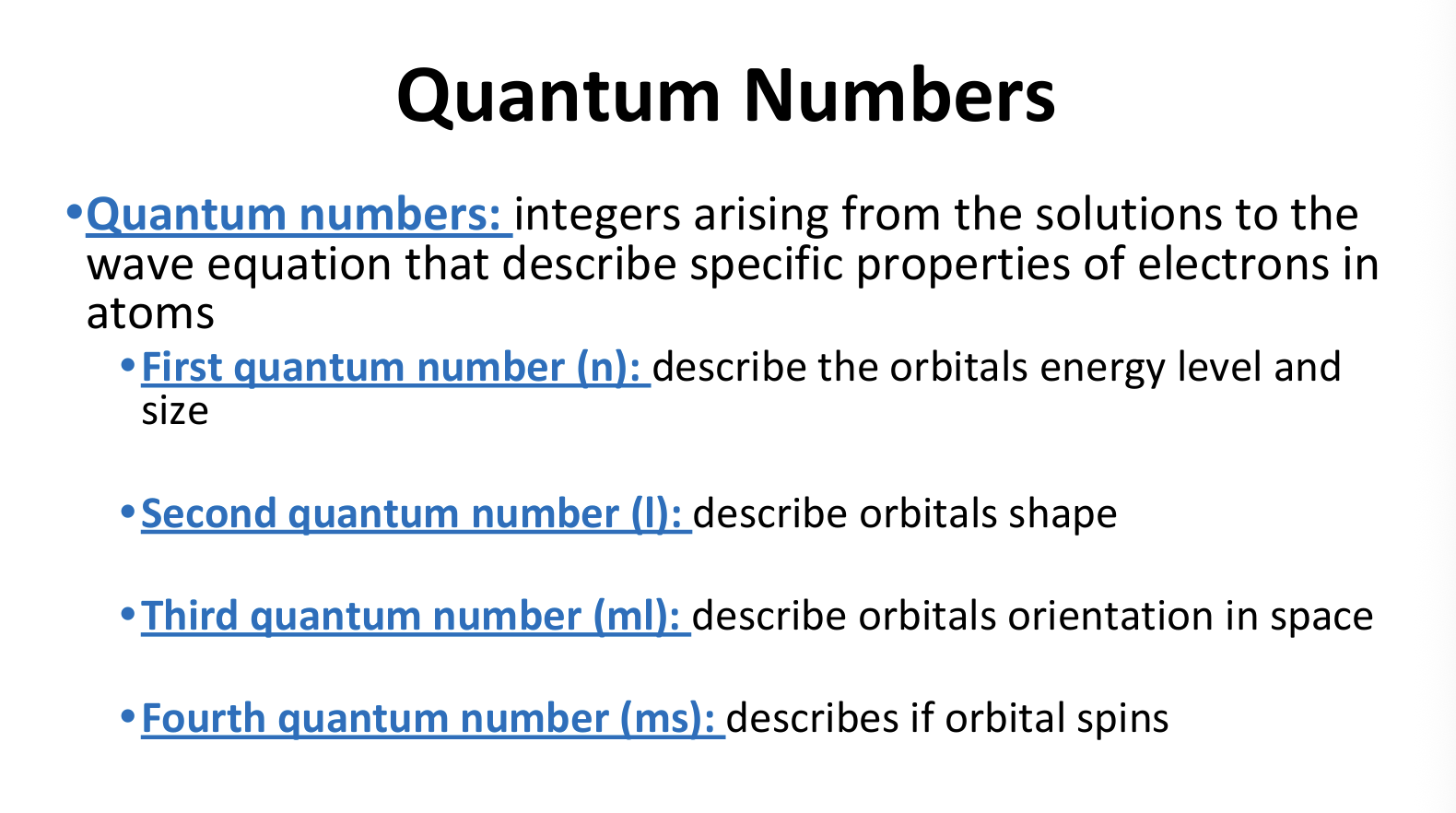
What are Quantum Numbers?
2
New cards
Values of l

3
New cards
Values of ml

4
New cards
Formula to Determine Max Number of Electrons
2N²
5
New cards
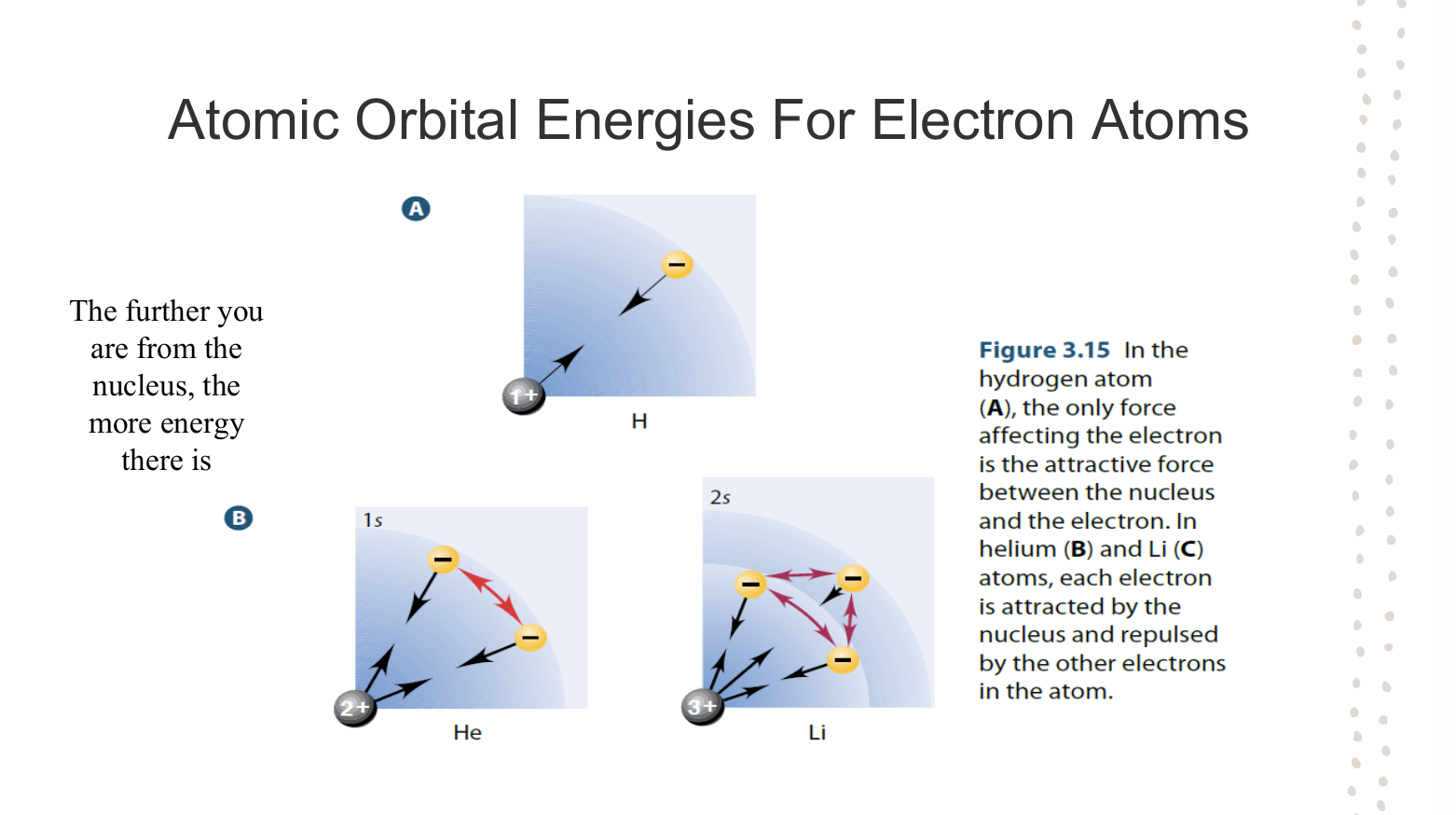
Presence of Electrons and Overall Energy Level

6
New cards
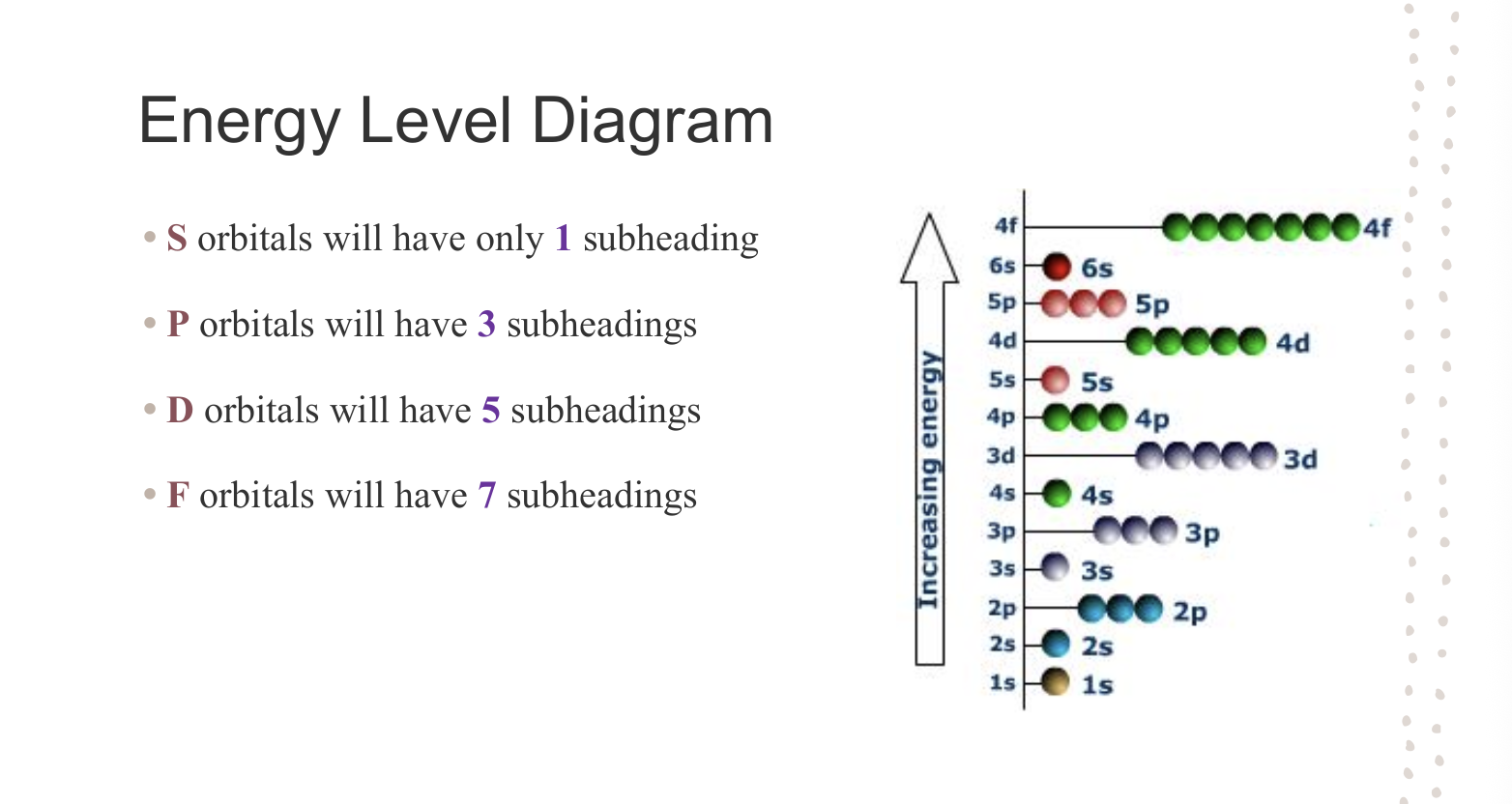
Energy Level Diagrams - Orbital Subheadings
7
New cards
Rules/Principals of Energy Diagrams

8
New cards
Molecular Orbitals for S, P, D, F

9
New cards
Periodic Trends - Atomic Radii
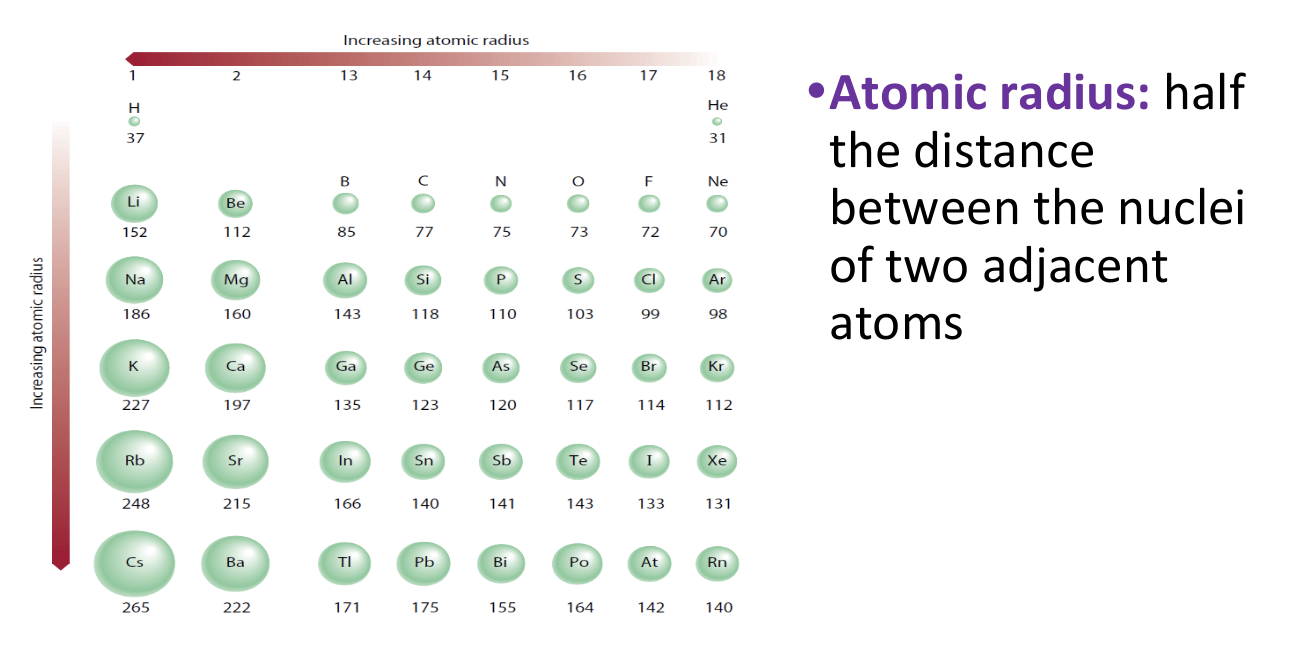
10
New cards
Factors Affecting Atomic Radius

11
New cards
Periodic Trends - Ionization Energy
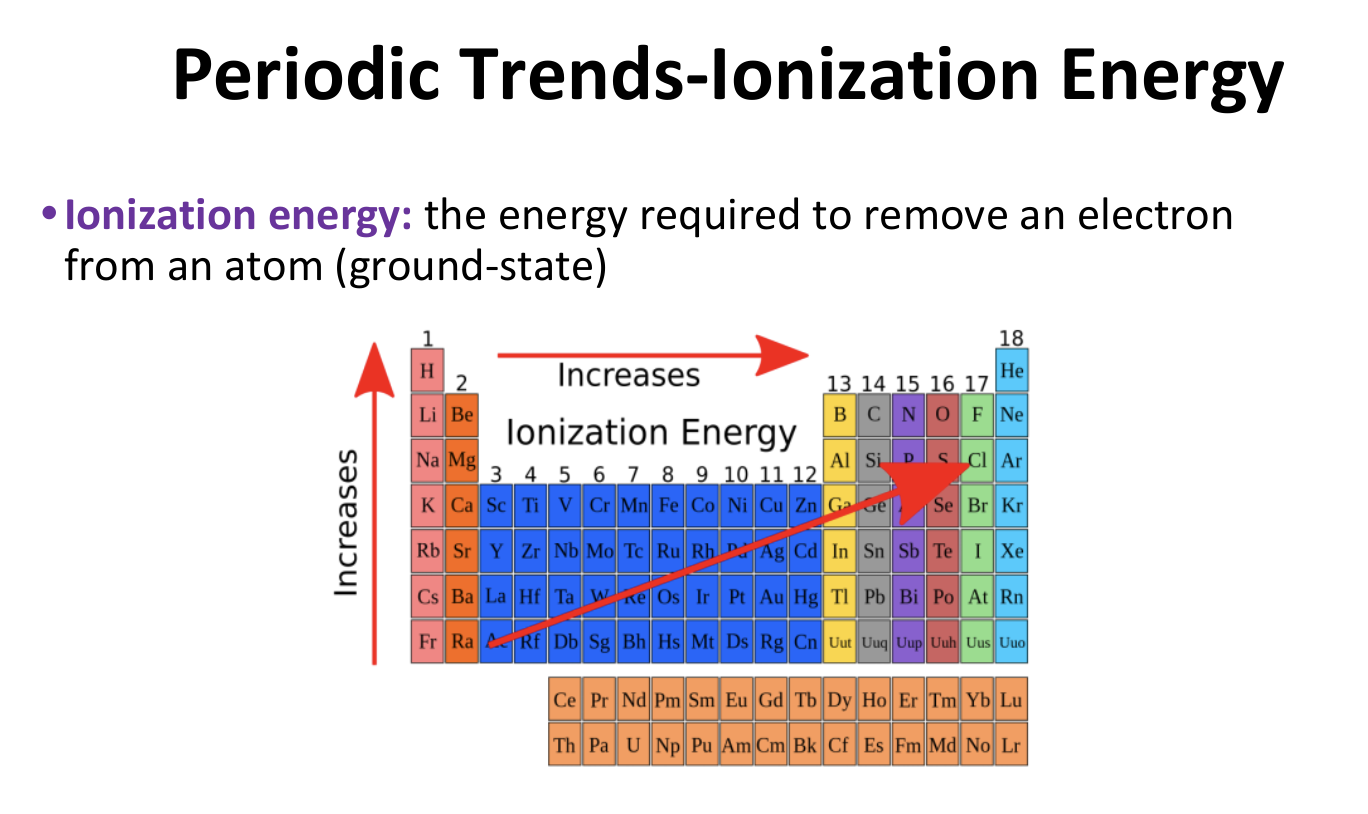
12
New cards
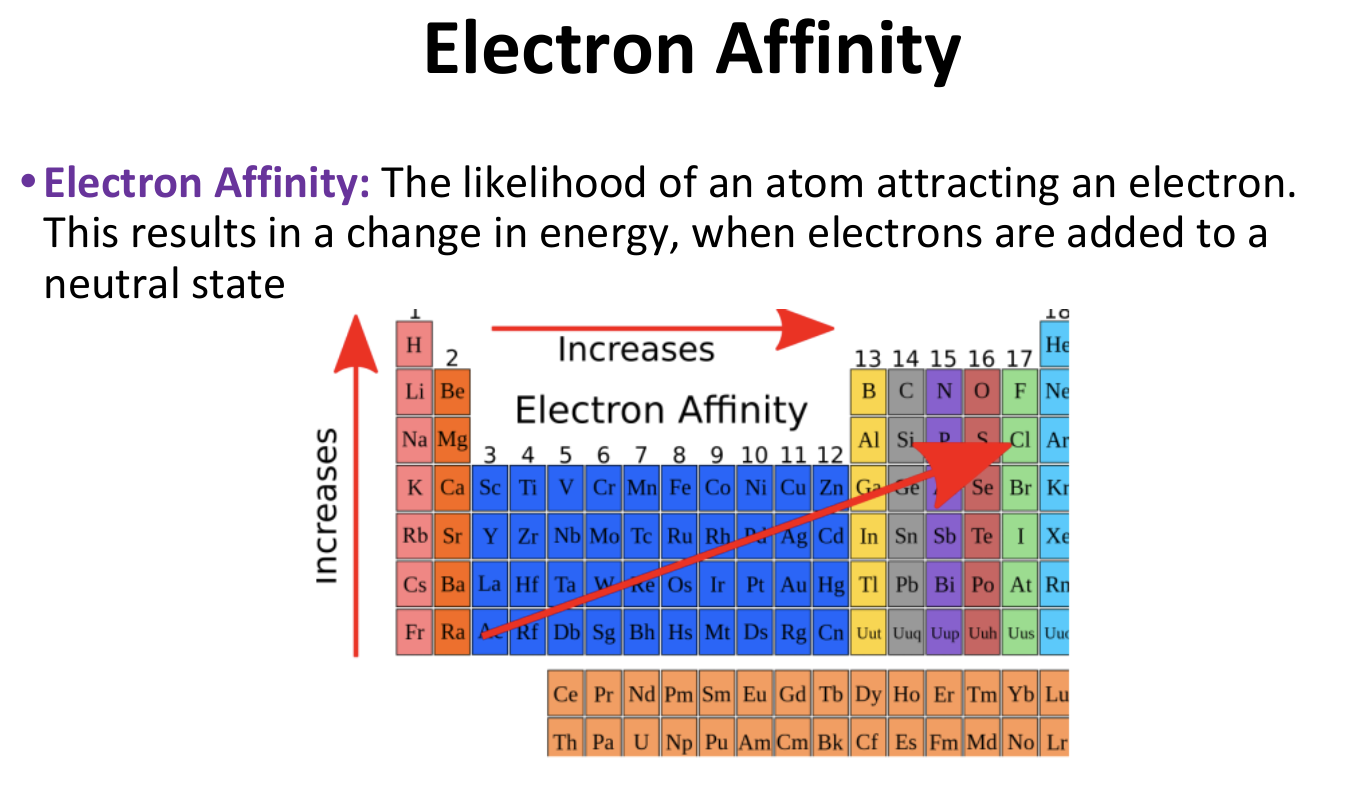
Periodic Trends - Electron Affinity