C4.2 Transfers of Energy and Matter
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
ecosystem
ecological systems composed of all organisms in an area together with their abiotic environment
system
a set of interacting or interdependent components
open systems - resources (including chemical substances and energy) can enter or exit
closed systems - energy can enter or exit, chemical resources cannot be removed or replaced
amount of energy reaching Earth’s surface in sunlight and amount harvested by producers varies around the world
high sunlight intensity, little energy harvested (few producers) vs. low sunlight intensity, more energy harvested by producers
reduced light penetration in marine and freshwater ecosystems - contain organisms and non-living matter, turbidity (suspended clay/silt, dense phytoplankton populations)
energy in cave systems
streams entering cave may bring dead or organic matter, providing energy supply
archaebacteria (producers) gain energy from chemical reactions that have methane, sulfides or any other inorganic compounds such as substrates, energy used to synthesize carbon compounds in chemosynthesis metabolism
microscopic invertebrates feed on biofilms of chemosynthetic archaebacteria, fed on by other invertebrates
food chain
sequence of organisms where each feeds on the previous one
producers - absorb sunlight using chlorophyll and photosynthetic pigments, convert light to chemical energy, used to fix energy in carbon compounds
consumers - obtain energy from carbon compounds in organisms on which they feed
arrows indicate direction of energy flow
different from food webs
saprotrophs
type of decomposer, bacteria and fungi
secrete digestive enzymes into dead organic matter, digest externally, absorb products of digestion, including sugars and amino acids
break down complex insoluble carbon compounds into simpler soluble ones, gradual breakdown of solid structures, recycling chemical elements
carbon compounds required to fulfill organisms’ nutritional requirements
amino acids for protein synthesis
sugars for energy supply, synthesis of polysaccharides
fatty acids for energy supply and constructing membranes
organic bases for synthesizing nucleic acids during DNA replication and transcription
steroids, many other groups of carbon compounds
autotrophs
self-feeding organisms, able to make all carbon compounds themselves
use CO2 or hydrogen carbonate (HCO3-) as carbon source, nitrate, phosphate, other simple inorganic substances as sources of other elements
external energy source (light or chemical reactions) needed to carry out anabolic reactions to build carbon compounds from simple inorganic substances
photoautotrophs or chemoautotrophs
photoautotrophs
use external light energy
fusion reactions in the sun generate vast amounts of energy in the form of electromagnetic radiation
small portion absorbed by photosynthesis in plants, eukaryotic algae, cyanobacteria
chemoautotrophs
use exothermic inorganic chemical reactions (prokaryotes, bacteria, archaebacteria)
substrate in reduced state is oxidized, releasing energy which is used to synthesize carbon compounds, producing their own sugar, amino acids, etc.
iron-oxidizing bacteria as an example of a chemoautotroph
*
heterotrophs
organisms who fulfill nutritional requirements by obtaining carbon compounds from other organisms, use products of digestion to build large complex carbon compounds
assimilation
process of absorbing carbon compounds into cells
molecules must be small and soluble enough to pass across cell membranes, large compounds must be digested before absorption
divisions of heterotrophs based on digestion of food (internal vs. external)
saprotrophs - grow into/across surface of food, secrete hydrolytic enzymes to digest food externally
consumers - ingest food
multicellular consumers - swallow food, mix with enzymes from digestive glands, internal digestion
unicellular consumers such as paramecium - take food into cells by endocytosis, digested inside phagocytic vacuoles, products of digestion absorbed from vacuoles into cytoplasm
vital activities requiring ATP
synthesizing large molecules
pumping molecules or ions by active transport
moving structures within cells
maintaining constant body temperature
ATP produced by cellular respiration, carbon compounds oxidized to release energy
trophic levels
grouping of organisms based on how they obtain energy and carbon compounds
construction of energy pyramids
x
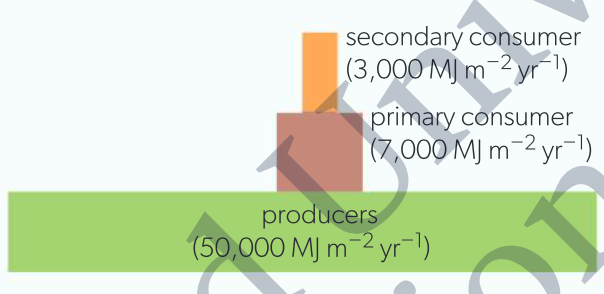
reductions in energy availability at each successive stage in food chains due to large energy losses between trophic levels
incomplete consumption - energy in dead parts of organisms passes to saprotrophs or detritus feeders rather than organisms in next trophic level, energy lost from food chain
incomplete digestion - not all food digested and absorbed, indigestible material egested in feces, energy passed to saprotrophs or detritus feeders
cell respiration - substrates oxidized to CO2 and water, releasing energy, waste products which cannot supply energy to next trophic level
smaller energy flow from each trophic level due to smaller amounts of energy-containing substances
with each trophic level, more energy per unit of biomass
heat loss to environment in both autotrophs and heterotrophs
all organisms contain some chemical energy to heat, maintaining constant body temperature or as a side effect of activity
second law of thermodynamics - energy transfers are never 100% efficient, not all energy from oxidation of carbon compounds in cell respiration transferred to ATP, remainder converted to heat when ATP used in cell activities
heat lost to abiotic environment, heat passes from hotter to cooler bodies and cannot be recycled
restriction in the number of trophic levelsq
so much energy lost at each step of the food chain, less energy available to each successive trophic level, not enough to support another trophic level
production in ecosystems
accumulation of carbon compounds in biomass, accumulation when living organisms grow and reproduce, vary in different biomes
primary producers
autotrophs, synthesize carbon compounds from simple substances
gross primary production (GPP)
total biomass of carbon compounds made in plants by photosynthesis
net primary production
gross primary production (GPP) minus biomass due to respiration of plant; amount of biomass available to consumers
secondary production
accumulation of carbon compounds in biomass by heterotrophs
lower per unit area than primary production, loss of biomass with every trophic level due to cellular respiration
pool
reserve of an element, organic or inorganic (ex. CO2 in atmosphere as inorganic pool of carbon)
flux
transfer of the element from one pool to the other: photosynthesis, feeding, respiration
ecosystems as open systems
both matter and energy can enter or exit, carbon enters/exits as carbon dioxide through cell respiration or photosynthesis
carbon sink - photosynthesis exceeds respiration, net uptake of carbon
carbon source - respiration exceeds photosynthesis, net release of carbon
decomposition
saprotrophs digest dead organic matter, release carbon as carbon dioxide due to respiration
sequestration
removal of carbon from carbon cycle (ex. peat turns into coal, carbon in coal removed from carbon cycle for millions/hundreds of millions of years)
natural gas and oil as carbon sinks
formed over past 550 million+ years
deep burial of partially decomposed organic matter under sediments
high temperature caused chemical changes, produced oil and natural gas trapped under porous rock
coal as a carbon sink
formed 325-250 million years ago
accumulation of wood and other plant matter in swamps, buried under other sediments
peat as a carbon sink
formed mostly in the last 10,000 years
incomplete decomposition of dead plant matter due to acidic and anaerobic conditions in waterlogged bogs and swamps
biomass as a carbon sink
past few thousand years or more recent
plant biomass derived from photosynthesis, transferred along food chains to animal biomass
combustion
burning in air, carbon sinks transformed into carbon sources as carbon dioxide is produced and released into the atmosphere
flashpoint - specific ignition temperature which must be reached for combustion to occur
carbon balance
sequestration and photosynthesis vs. combustion and respiration
Mauna Loa Observatory, Hawaii
measures atmospheric carbon dioxide concentrations, results helped plot Keeling curve (Charles Keeling)
Keeling Curve trends
annual fluctuations
long-term trend - annual increase not completely reversed by December, concentration at end of year is higher; overall increase 1959-onwards due to anthropogenic factors
causes of annual fluctuation in CO2 emission
CO2 concentrations increase October-May, decrease May-October due to global imbalances in rates of photosynthesis and respiration
photosynthesis higher overall in northern hemisphere summer, plants over most of earth’s land surface are in main growth season
release of oxygen in photosynthesis
one oxygen atom removed from each of two water molecules, oxygen atoms linked by double covalent bond using energy derived from light
catalyzed by oxygen-evolving complex at center of photosystem II, developed in archaebacteria
chemical elements needed by all living organisms
carbon, hydrogen, oxygen
nitrogen, phosphorus
approximately 15 other elements
inorganic nutrients obtained from abiotic environment
nutrient cycles
recycling of chemical elements needed by organisms in an ecosystem