Chemistry - Physical Chemistry
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
How has the understanding of atom structure changed?
1661 - Robert Boyle proposed that there were some substances that could not be made simpler.
1803 - John Dalton suggested elements were composed of indivisible atoms; all atoms of a particular element had the same mass, and atoms of different elements had different masses. Atoms could not be broken down.
1896 - Henri Becquerel discovered radioactivity; atoms were not indivisible as particles came from atoms. Shortly after, J. J. Thomson discovered the electron, which had a negative charge, suggesting a positive charge elsewhere; proposed the plum pudding model.
1911 - Ernest Rutherford found that an atom was mostly empty space with a dense positive nucleus.
What are the subatomic particles found in an atom called, and what are their properties?
Protons: 1.673×10⁻²⁷ kg, +1.602×10⁻¹⁹ C, located in the nucleus.
Neutrons: 1.675×10⁻²⁷ kg, 0 charge, in the nucleus.
Electrons: 0.911×10⁻³⁰ kg, -1.602×10⁻¹⁹ C, orbiting the nucleus.
What is the relative charge and relative mass of electrons protons and neutrons?
Protons - relative charge +1, relative mass 1
2. Neutrons - relative charge 0, relative mass 1
Electrons - relative charge -1, relative 1/1840
How are the subatomic particles in an atom arranged?
The protons and neutrons are in the centre of the atom, held together by a force called the strong nuclear force. This is much stronger than the electrostatic forces of attraction that hold electrons and protons together in the atom, so it overcomes the repulsion between the protons in the nucleus. It acts only over very short distances, that is, within the nucleus.
The nucleus is surrounded by electrons. Electrons are found in a series of levels, sometimes referred to as orbits or shells, which get further and further away from the nucleus.
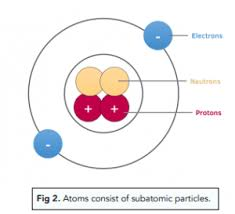
What is the atomic number of an element (Z)?
The number of protons in one atom of that element.
How many electrons are there in an element compared to its atomic number?
The number of electrons in an element are the same as its atomic number
What is the atomic mass number (A) of an element?
The number of neutrons + the number of protons in the nucleus of an atom of that element.
What are isotopes? What are their properties? (3)
Atoms with the same number of protons but different number of neutrons.
Different isotopes of the same element react the same due to the same electronic configuration
Atoms of different isotopes of the same element vary in mass number because they have a different number of neutrons in their nuclei.
What is the general equation for the number of electrons in a particular shell?
2n2 where n is the number of shells
What is a mass spectrometer used for?
Helps determine the relative atomic masses of a sample.
What happens in a time of flight mass spectrometer? (6)
Vacuum
Ionisation
Acceleration
Ion drift
Detection
Data Analysis
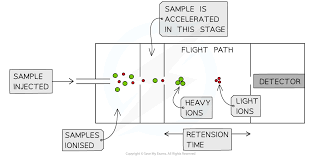
What happens in the vacuum stage of the (TOF) mass spectrometry?
The whole apparatus is kept under a high vacuum to prevent the ions that are produced colliding with molecules from the air.
What happens in the ionisation stage of the (TOF) mass spectrometry? (2)
Ionisation - Two types -
Electrospray ionisation, the sample is dissolved in a volatile solvent and forced through a fine hollow needle that is connected to the positive terminal of a high voltage supply. This produces tiny positively charged droplets that have gained a proton from the solvent. The solvent evaporates from the droplets into the vacuum and the droplets get smaller and smaller until they may contain no more than a single positively charged ion.
In electron impact, the sample is vaporised and high energy electrons are fired at it from an electron gun, which is a hot wire filament with a current running through it that emits beam of high energy electrons. This usually knocks off one electron from each particle forming a 1+ ion.
X(g) + e- → X+(g) + 2e-
What happens in the acceleration stage of a (TOF) mass spectrometer?
The positive ions are attracted towards a negatively charged plate and accelerate towards it. Lighter ions are more highly charged ions achieve a higher speed.
What happens in the Ion drift stage of (TOF) mass spectrometry?
The ions pass through a hole in the negatively charged plate, forming a beam and travel along a tube, called the flight tube, to a detector.
What happens in the detection stage of a (TOF) mass spectrometer?
When ions with the same charge arrive at the detector, the lighter ones are first as they have higher velocities. The flight times are recorded. The positive ions pick up an electron from the detector which causes a current to flow.
What happens during the Data Analysis stage of (TOF) mass spectrometry?
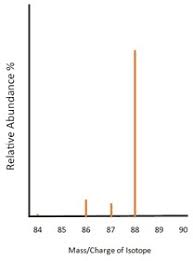
The signal from the detector is passed to a computer which generates a mass spectrum like those in the image.
What are the different resolutions of mass spectrometry?
High resolution - relative atom masses to five decimal places of an atmic mass unit.
Low resolution - One decimal point
How do you read a mass spectrometer results graph?
Y axis - relative abundance %
X axis - mass/charge ratio
How can mass spectrometry be used to identify elements?
All elements have a characteristic pattern that shows the relative abundances of their isotopes.
How is a single electron shell sub-divided into?
s,p,d,f subshells
What are atomic orbitals?
An electron fills a volume in space called its atomic orbital
What is the shape of a s subshell?
A sphere
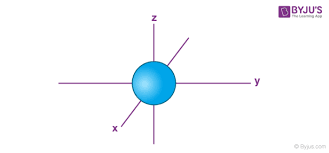
What is the shape of a p subshell?
Dumbell shape
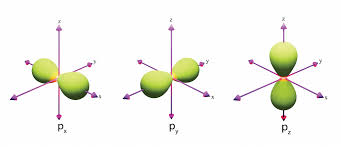
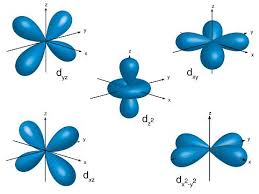
What is the shape of a d oprbital?
Dumbell with a ring around the centre or like a 4 leaf clover
How many electrons can each electron orbital hold?
s-orbitals can hold up to 2 electrons
p-orbitals can hold up to 6 electrons
d-orbital can hold up to 10 electrons
f-orbital can hold up to 14 electrons
Explain the spin property of electrons
Two electrons in the same orbital must have opposite spins
The electrons are usually represented by arrows pointing up or down to show the different directions of spin
What are the rules associated with allocating electrons to atomic orbitals? (3)
Atomic orbitals of lower energy are filled first - so the lower main level is filled first and, within this level, sub levels of lower energy are filled first.
Atomic orbitals of the same energy fill singly before pairing starts. This is because electrons repel each other.
No atomic orbital can hold more than two electrons
What is ionisation energy?
The energy required to remove a mole of electrons from a mole of atoms in the gaseous state and is measured in KJ mol-1
What are successive ionisation energies?
The amount of energy required to remove an electron, then the next and so on.
Why might the first ionisation energies of elements across a period not be regular?
The electrons might be further away even though they have a higher nuclear charge.
What are the three main factors that impact ionisation energy?
Atomic radius - the larger the radius, the lower the electrostatic forces of attraction between the electron and the nucleus so less energy is needed to remove the electron.
Nuclear Charge - The higher the nuclear charge the higher the electrostatic forces of attraction between the nucleus and the electrons so more energy is required to remove them.
Electron Shielding - Inner most electrons repel and block the attraction between the nucleus and the outer most electrons.
How might paired electrons make it easier to lose an electron?
The energy required to lose an electron in phosphorus is more than sulfur, this is because in sulfur there are paired electron on the other shell meaning the repulsion between these two electrons makes it easier to remove one of the electrons.
Explain the trend in ionisation energies down a group in the Periodic Table.
There is a general decrease in first ionisation energy going down the group. This is because the outer electron is in a main level that gets further from the nucleus in each case. Going down a group, the nuclear charge increases. At first sight you might expect that this would make it more difficult to remove an electron. However, the actual positive charge felt by an electron in the outer shell is less than the full nuclear charge. This is because of the effect of the inner electrons shielding the nuclear charge.
Explain the trend of ionisation energies across period 3.
General increase due to increasing nuclear charge.
From Mg-Al the energy decrease instead of increases because the other electron in aluminium is in a 3p orbital which is of a slightly higher energy than the 3s orbital.
There is a drop from P-S because of electron-electron repulsion as in Sulfur there is a pair of electrons in one orbital meaning it is easier to lose one of these electrons as they repel eachother.
What is the relative atomic mass Ar of a substance?
The weighted average mass of an atom of an element, taking into account its naturally occurring isotopes, relative to 1/12 the relative atomic mass of an atom of carbon-12.
What is the equation for relative atomic mass Ar?
(average mass of one atom of an element)/(1/12 mass of one Carbon-12)
What does relative molecular mass Mr mean?
The mass of that molecule compared to 1/12 the relative atomic mass of an atom of carbon-12
What is the equation for relative molecular mass Mr?
(average mass of one molecule)/(1/12 mass of one atom of carbon-12)
Why are relative formal masses used for ionic compounds?
Because they don’t exist as molecules. However, this has the same symbol Mr
What is Avogadro’s constant?
The number of atoms in 12g of carbon-12
What is a mole?
The amount of substance that contains 6/022×1023 particles is called a mole.
What is the equation linking number of moles, mass and mass?
(number of moles n) x (mass of 1 mole M (g)) = mass m (g)
What is the equation linking concentration, volume and moles?
(number of moles n) = (concentration c (mol dm-3)) x (volume V (dm-3))
What is Boyle’s Law - equation linking volume and pressure?
pressure P x volume V = constant
What is Charle’s law - volume and temperature
volume V ∝ temperature T and V/T=constant
the volume is proportional to the temperature as long as the pressure remains constant
What is Gay-Lussac’s law - pressure and temperature
pressure P ∝ temperature T and P/T=constant
What is the ideal gas equation?
PV=nRT
P - Pressure - Pa
V - Volume - m3
n - Number of moles
R - gas constant - (JK-1 mol-1)
T - Temperature - K