Organic Chemistry Exam 1
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
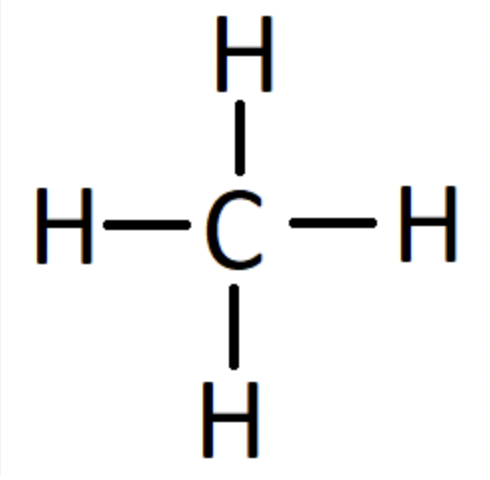
methane
CH4
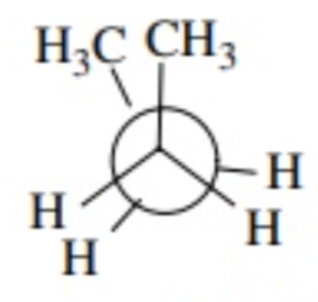
ethane
C2H6 - CH3CH3
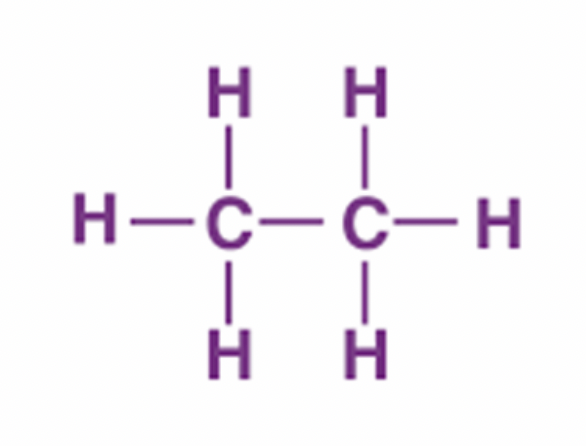
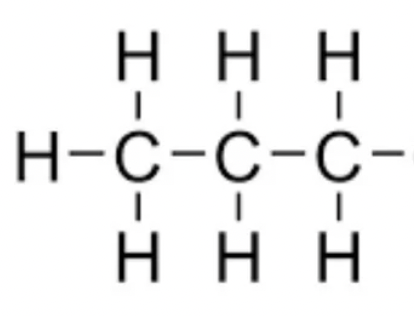
propane
C3H8 - CH3CH2CH3
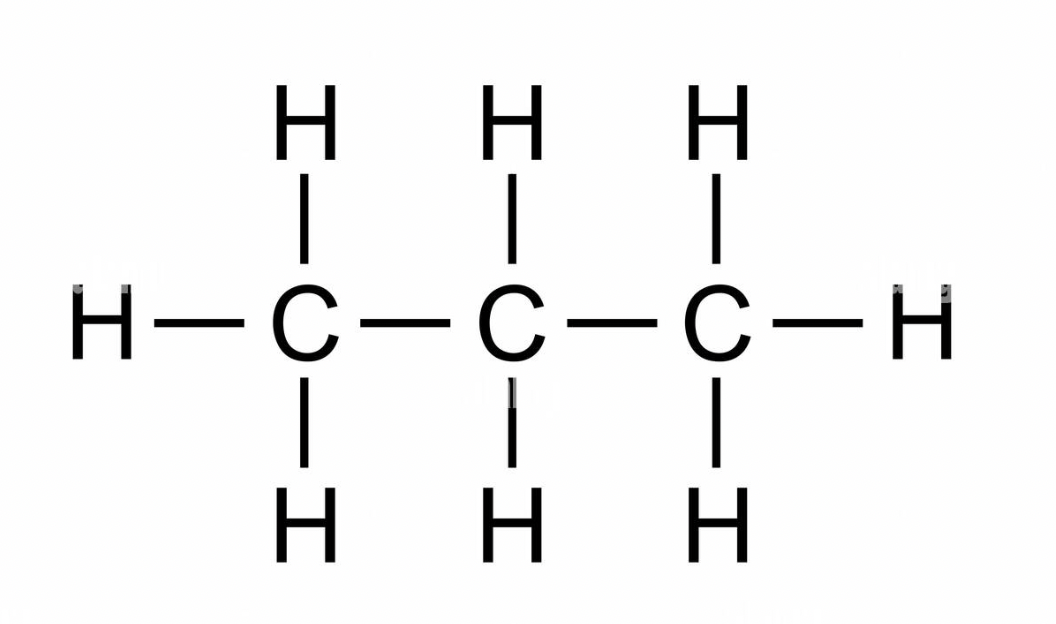
butane
C4H10 - CH3(CH2)2CH3
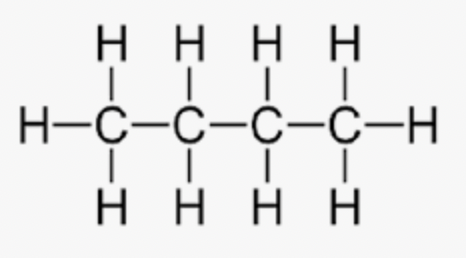
pentane
C5H12 - CH3(CH2)3CH3
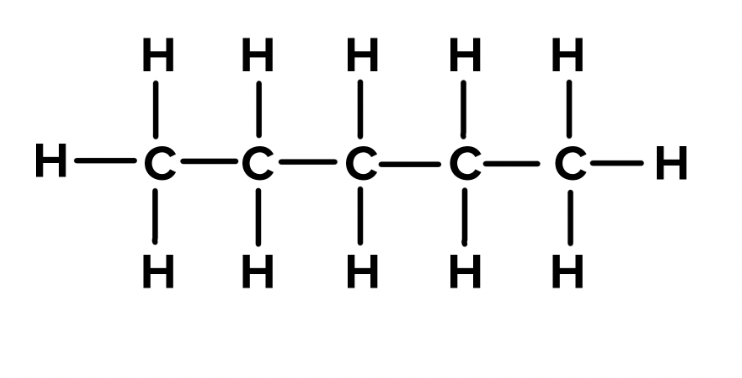
hexane
C6H14 - CH3(CH2)4CH3
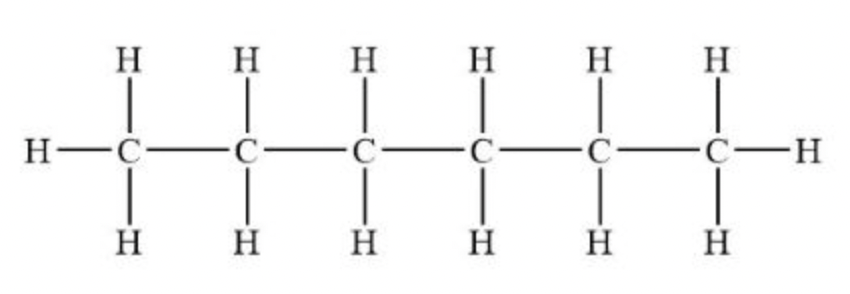
heptane
C7H16 - CH3(CH2)5CH3
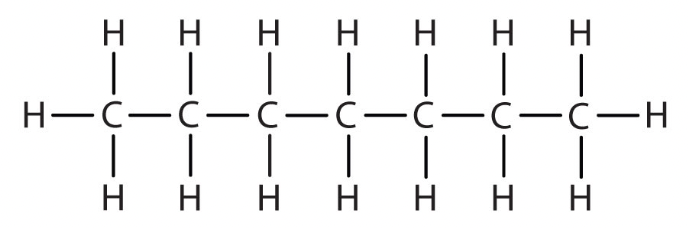
octane
C8H18 - CH3(CH2)6CH3
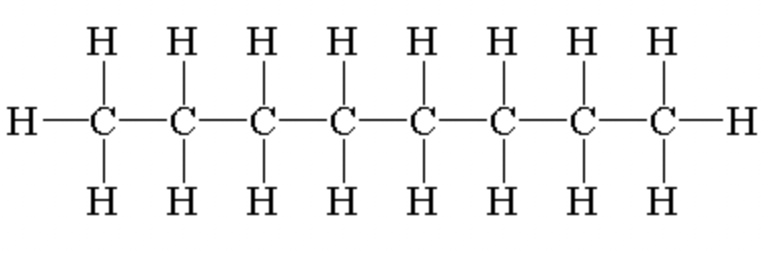
nonane
C9H20 - CH3(CH2)7CH3
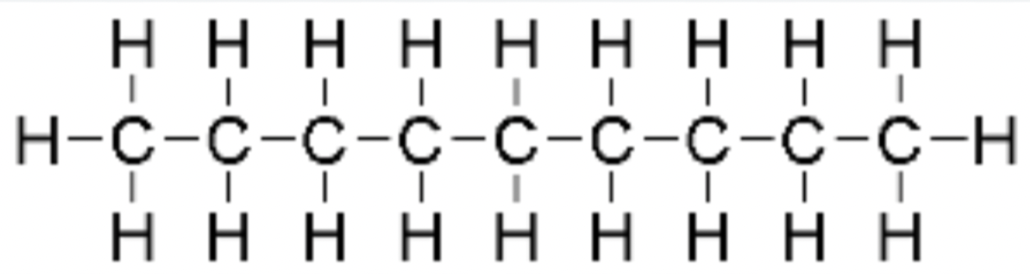
decane
C10H22 - CH3(CH2)8CH3
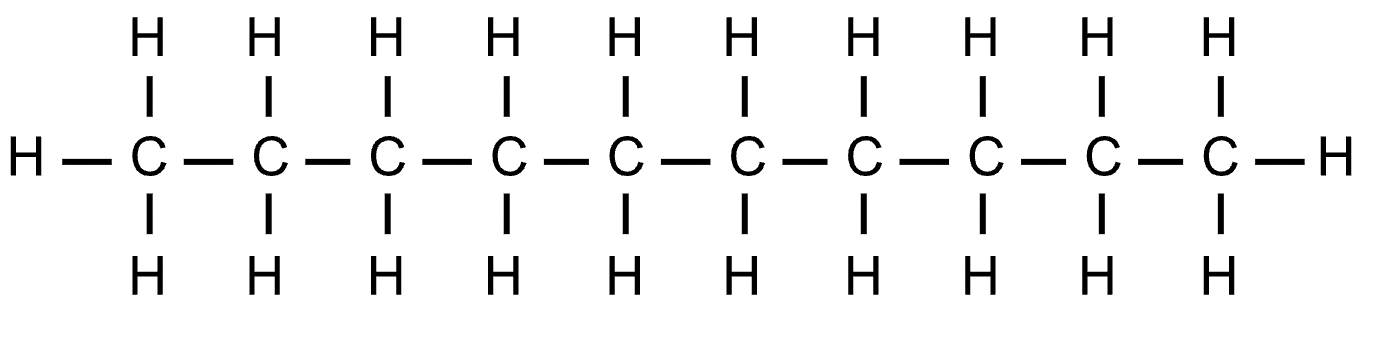
undecane
C11H24 - CH3(CH2)9CH3
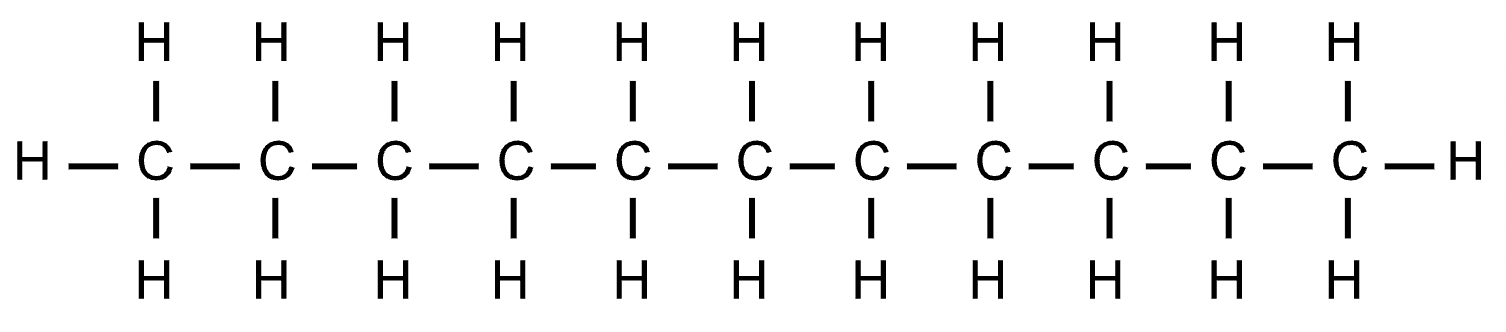
dodecane
C12H26 - CH3(CH2)10CH3
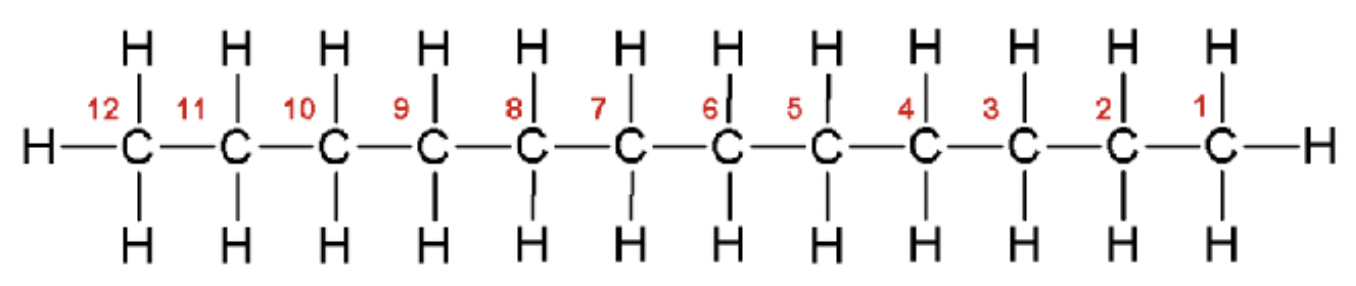
methyl (Me)
alkyl group
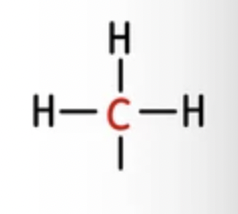
ethyl (Et)
alkyl group
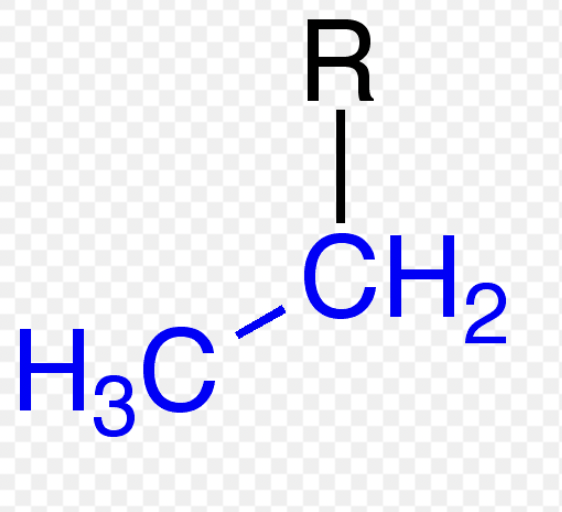
n-propyl (n-Pr)
alkyl group
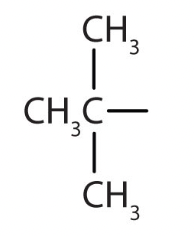
isopropyl (iPr)
alkyl group
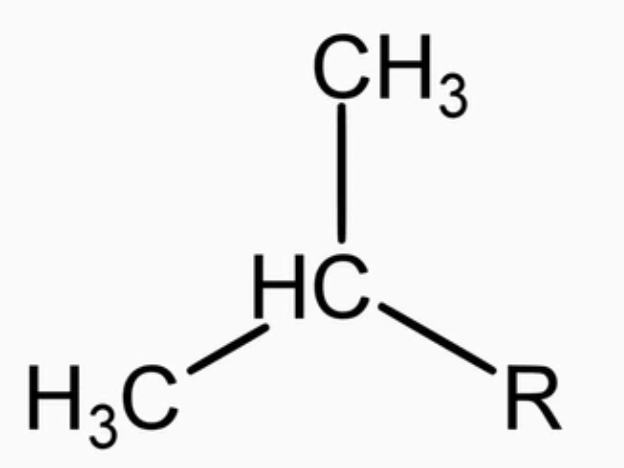
n-butyl (n-Bu)
alkyl group
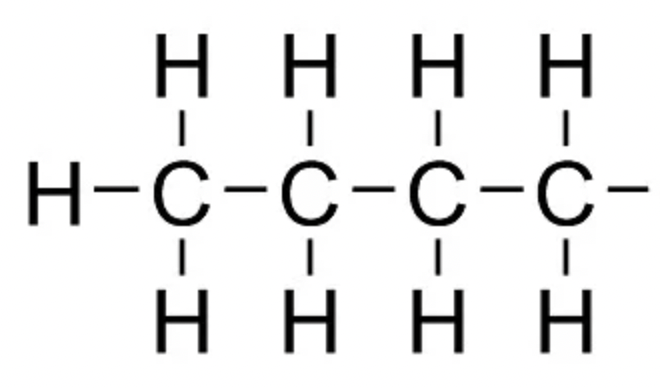
isobutyl (iBu)
alkyl group
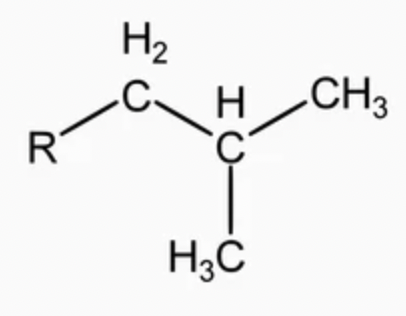
sec-butyl (s-Bu)
alkyl group
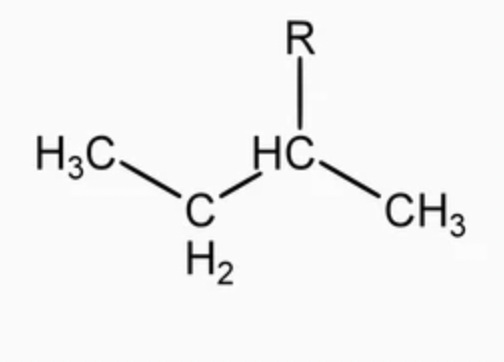
tert-butyl (t-Bu)
alkyl group
hydrocarbon
compounds that contain carbon and hydrogen atoms with single covalent bonds
saturated
all carbon atoms are bonded to 4 other atoms via single sigma bonds (all carbon atoms are sp3 - hybridized)
unsaturated
one or more carbon atoms are bonded to atoms via double/triple (pi) bonds (one or more atoms are sp2/sp hybridized)
functional groups
an atom or a group of atoms that exhibits a characteristic (predictable) chemical behavior and behaves the same way chemically in every compound it is a part of
alkane
functional group
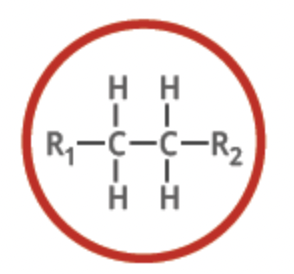
alkene
functional group

alchohol
functional group

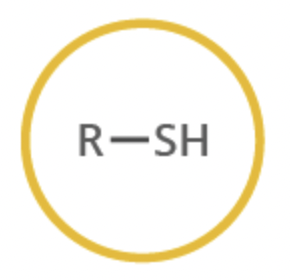
thiol
functional group
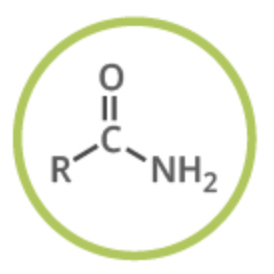
amide
functional group
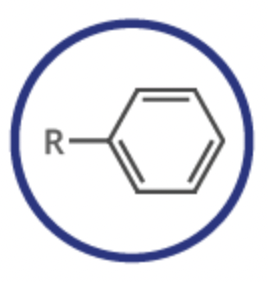
arene
functional group
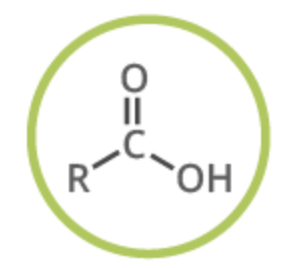
Carboxylic acid
functional group
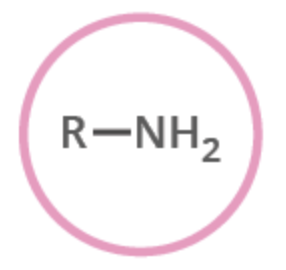
amine
functional group
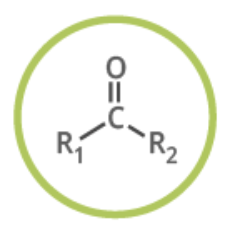
ketone
functional group
conformations
different molecular shapes or 3-D structures that result from rotation about single sigma bonds
torsional strain
resistance to rotation due to electronic repulsion between adjacent bonding electrons that increase energy (destabilization) resulting from eclipsed bonds on adjacent atoms
steric strain
electronic repulsion between two groups through space
cycloalkanes
saturated hydrocarbons where the carbon forms a ring
anti staggered
180 degreees
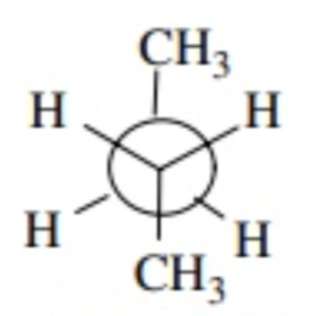
gauche staggered
60 degrees
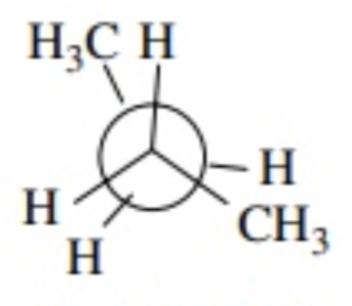
CH3/H eclipsed
120 degrees
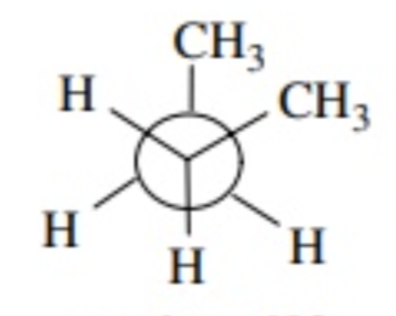
CH3/CH3 eclipsed
0 degrees