AQA A level Chemistry 3.2.4 Period 3 Elements
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Write the reaction equation for sodium with water. (2)
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
What are the observations when sodium reacts with water? (2)
- The reaction is exothermic.
- Gas is produced - effervescence
What is the product formed when sodium reacts with water? (1)
Colourless very alkaline solution
Write the reaction equation for magnesium with cold water. (2)
Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)
What are the observations when magnesium reacts with cold water? (2)
- Minor bubbles are formed.
- The reaction is slow.
What is the product formed when magnesium reacts with cold water? (1)
Sparingly soluble, weakly alkaline solution.
Write the reaction equation for magnesium with steam. (2)
Mg(s) + H2O(g) → MgO(s) + H2(g)
What are the observations when magnesium reacts with steam? (2)
- A white flame is produced.
- A white solid is formed during a fast reaction
What is the product formed when magnesium reacts with steam? (1)
Basic solid.
Why does Na react more readily with cold water than Mg? (1)
It takes less energy to lose one electron in sodium compared to two electrons in magnesium
Write the reaction equation for sodium with oxygen. (2)
2Na(s) + 1/2O2(g) → Na2O(s)
What are the observations when sodium reacts with oxygen? (3)
- Yellow flame (or orange).
- Yellow solid is formed.
- Reacts readily in air
Write the reaction equation for magnesium with oxygen. (2)
Mg(s) + 1/2O2(g) → MgO(s)
What are the observations when magnesium reacts with oxygen? (3)
- White flame.
- White solid is formed.
- Reacts readily in air.
Write the reaction equation for aluminium with oxygen. (2)
2Al(s) + 1 1/2O2(g) → Al2O3(s)
What is the observation when aluminium reacts with oxygen? (1)
Reacts slowly.
Write the reaction equation for silicon with oxygen. (2)
Si(s) + O2(g) → SiO2(s)
What is the observation when silicon reacts with oxygen? (1)
Reacts slowly
Write the reaction equation for phosphorus with oxygen. (2)
P4(s) + 5O2(g) → P4O10(s)
What are the observations when phosphorus reacts with oxygen? (3)
- White smoke.
- White flame.
- P4 ignites spontaneously in air, so it is stored in oil or under water.
Write the reaction equation for sulfur with oxygen. (2)
S(s) + O2(g) → SO2(g)
What are the observations when sulfur reacts with oxygen? (3)
- Blue flame.
- Choking gas (SO2).
- Reacts readily in air.
What is the structure of Na2O and MgO? (1)
Giant ionic lattice
What is the structure of Al2O3? (1)
Giant ionic lattice
What type of bonding is present in Al2O3? (1)
Ionic with covalent character
What is the structure of SiO2? (1)
Macromolecular
What type of bonding is present in SiO2? (1)
Strong covalent bonds
What is the structure of P4O10? (1)
Simple molecular
What type of intermolecular force is present in P4O10? (1)
Weak Van der Waals forces between molecules
What is the structure of SO3 and SO2? (1)
Simple molecular
What type of intermolecular force is present in SO3 and SO2? (1)
Weak Van der Waals forces between molecules
Which ionic oxides are basic? (2)
Na2O and MgO
Which oxide is amphoteric? (1)
Al2O3 (Aluminium oxide)
What does it mean for an oxide to be amphoteric? (1)
It can act as an acid or a base
Which oxides are acidic? (4)
SiO2 (Macromolecular), P4O10, SO2, and SO3 (Simple molecular oxides)
What is the reaction of Na2O with water? (1)
Na2O(s) + H2O(l) → 2NaOH(aq)
What is the pH of the solution formed when Na2O reacts with water? (1)
12-14 (Very alkaline)
What is the reaction of MgO with water? (1)
MgO(s) + H2O(l) → Mg(OH)2(aq)
What is the pH of the solution formed when MgO reacts with water? (1)
9-10 (Alkali but Mg(OH)2 is sparingly soluble)
Does Al2O3 react with water? (2)
- No, Al2O3 is insoluble
- Forms a protective layer on the surface of Al
What is the pH of Al2O3 with water? (1)
7 (Neutral)
Does SiO2 react with water? (1)
No, SiO2 is insoluble
What is the reaction of P4O10 with water? (1)
P4O10(s) + 6H2O(l) → 4H3PO4(aq)
What acid is formed in the reaction of P4O10 with water? (1)
Phosphoric acid (H3PO4)
What is the reaction of SO2 with water? (1)
SO2(g) + H2O(l) → H2SO3(aq)
What acid is formed in the reaction of SO2 with water? (1)
Sulfurous acid (H2SO3)
What is the reaction of SO3 with water? (1)
SO3(g) + H2O(l) → H2SO4(aq)
What acid is formed in the reaction of SO3 with water? (1)
Sulfuric acid (H2SO4)
Write the equations for the reactions of Na20(s) with acids (3)
Exothermic and slow reactions
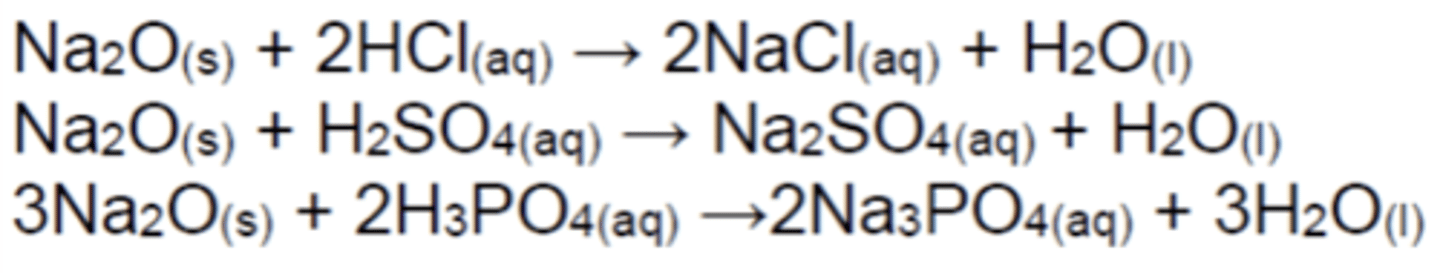
Write the equations for the reactions of MgO(s) with acids (3)
Slow reactions
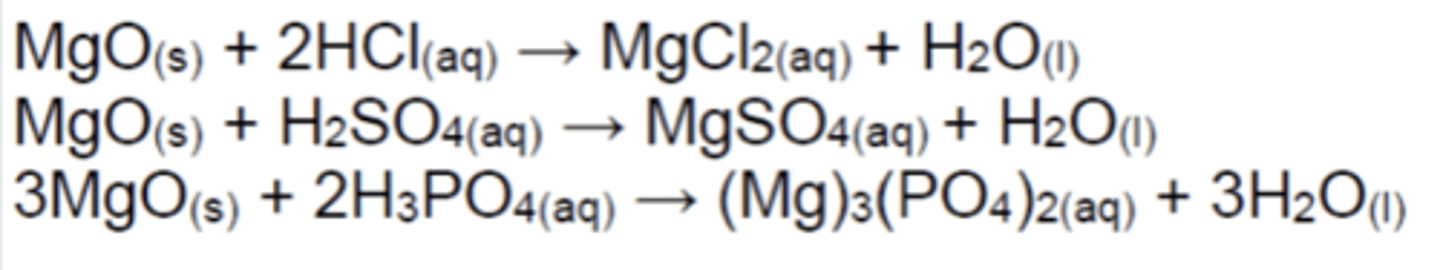
Write the equations for the reactions of Al2O3(s) with acids and bases (3)

Write the equations for the reactions of SiO2(s) with bases (3)
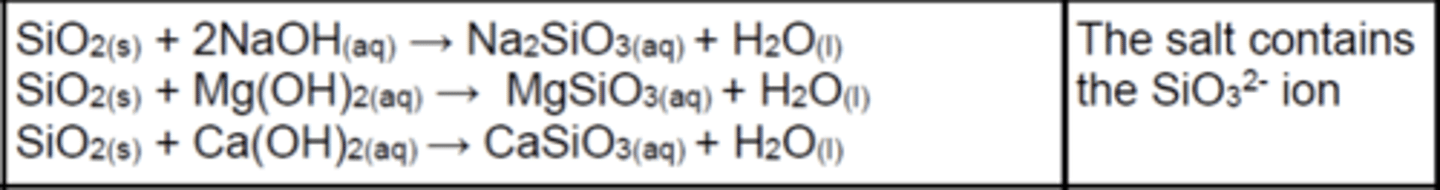
Write the equations for the reactions of P4O10(s) with bases (3)
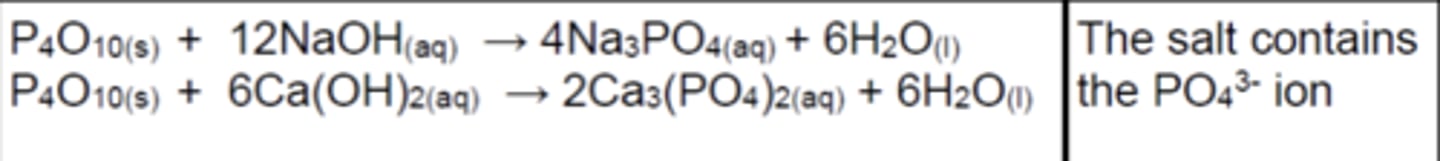
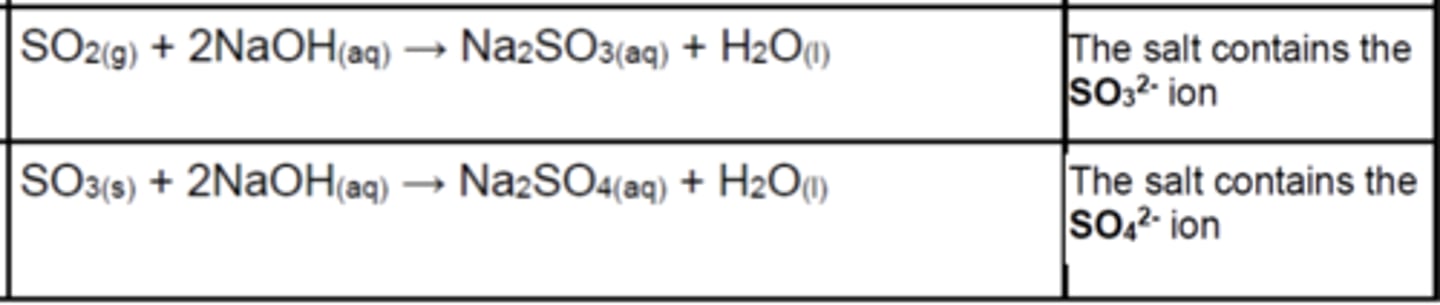
Write the equations for the reactions of SO2(s) and SO3 with bases (3)