Chemistry Mid-term (semester spring)
5.0(1)
Card Sorting
1/24
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
1
New cards
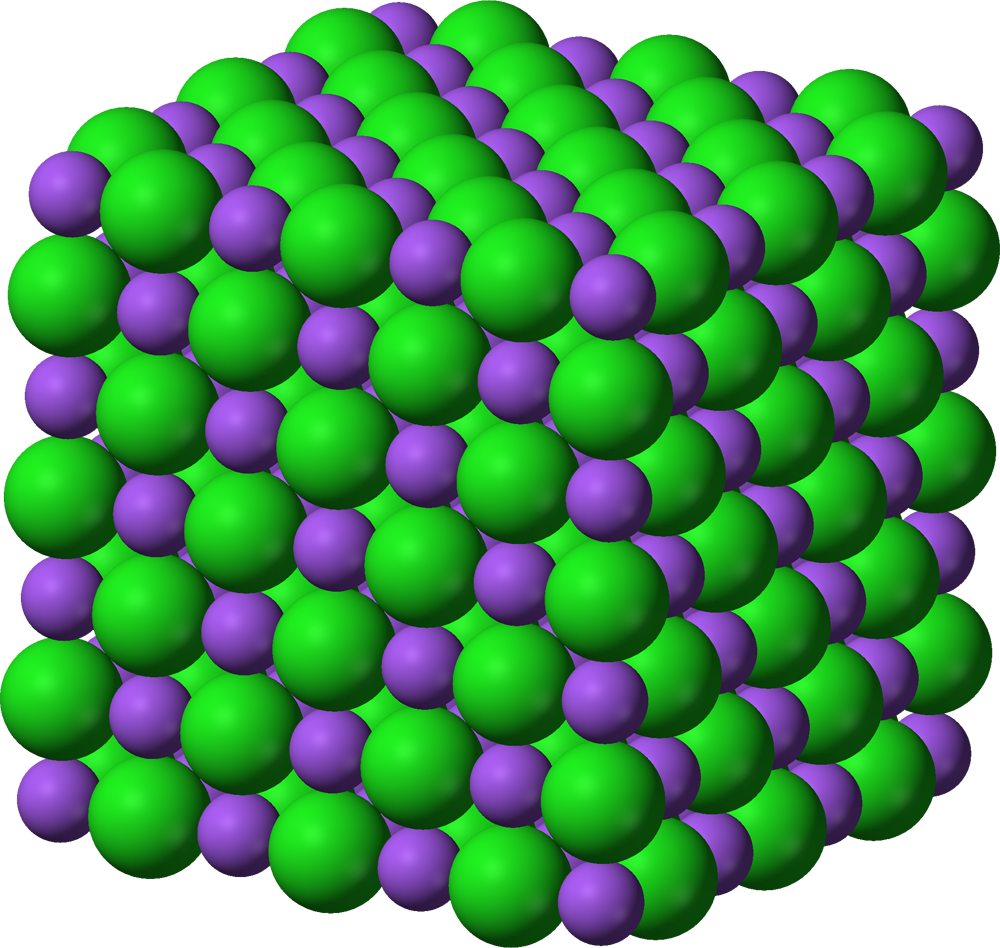
Crystal
Trillions of ions arranged in a repeating alternating pattern: Cation-anion-cation-anion-cation-anion…It could be the 3D-shaped structure of ionic bondings or metals.
2
New cards
Representative particles
The smallest unit in which a substance naturally occurs.
3
New cards
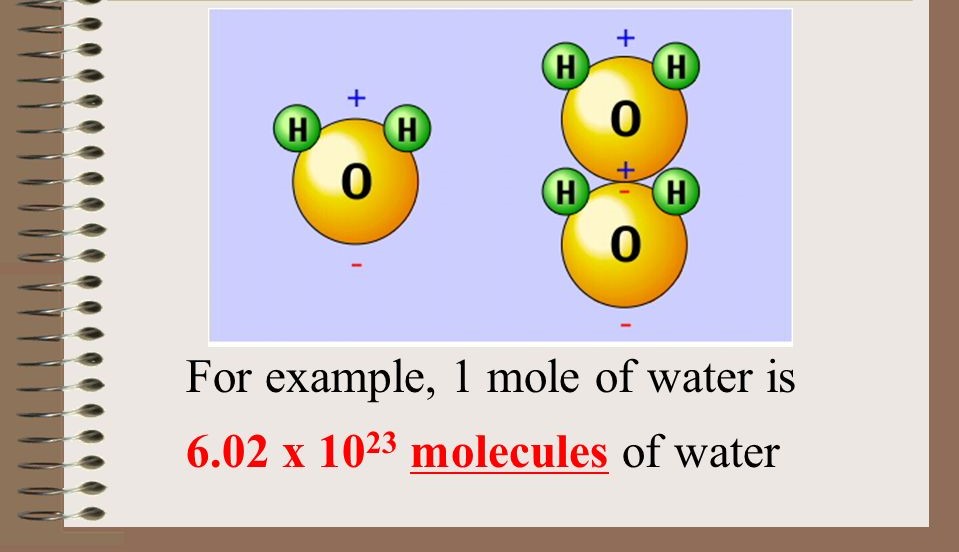
Mole
The SI unit for amount of substance: The amount of substance that contains the same number of representative particles as there are atoms in 12 g of carbon-12, i.e.,
6\.02 x 1023
6\.02 x 1023

4
New cards
Atom
The representative particle for elements is the ________.
5
New cards
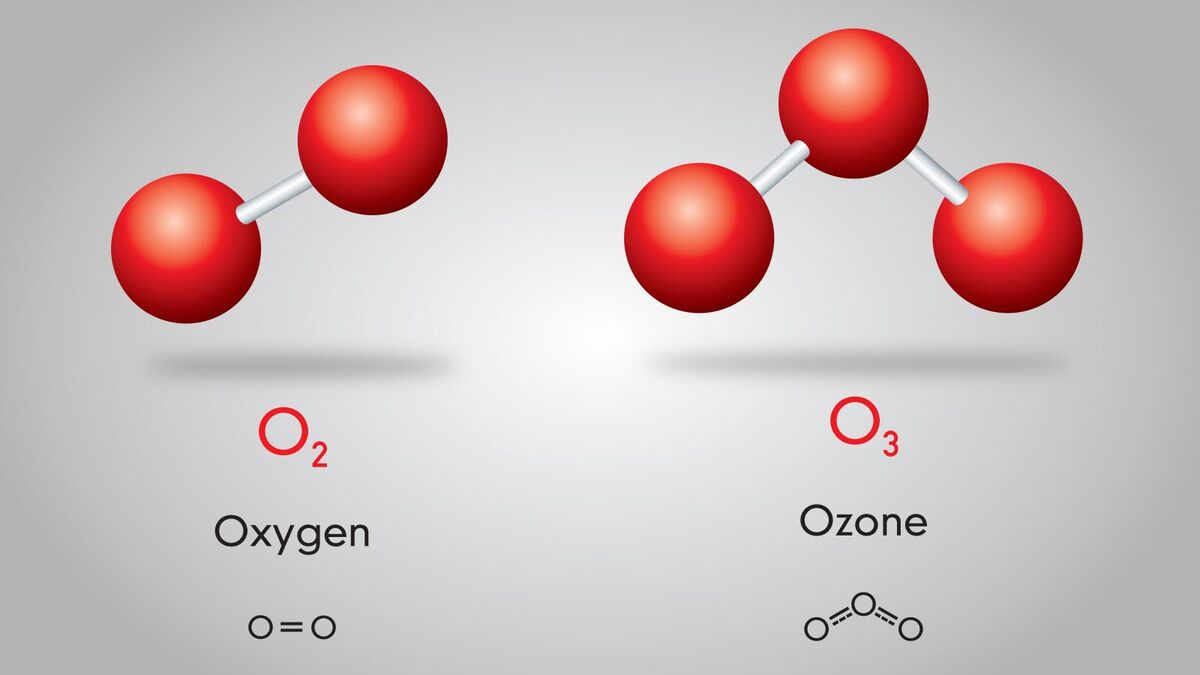
Molecule
Two or more atoms covalently bonded together (may be same type of atom, e.g., O2, or different types, e.g. H2O).
6
New cards
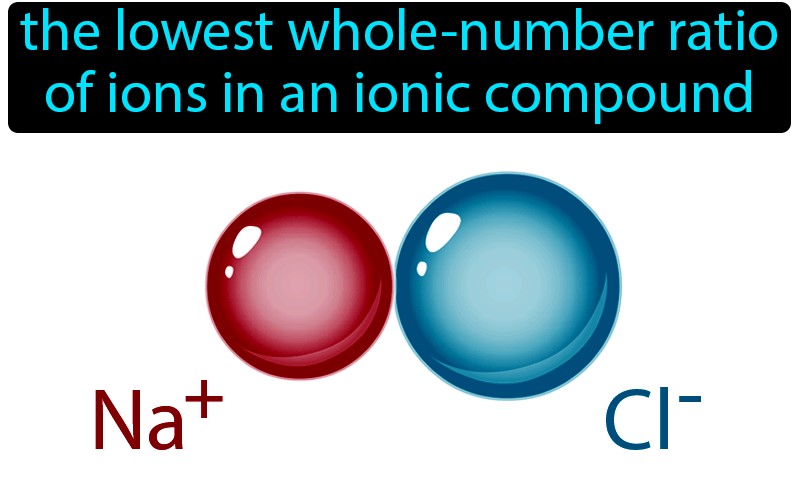
Formula unit
The representative particle for ionic compounds is the ________.
7
New cards
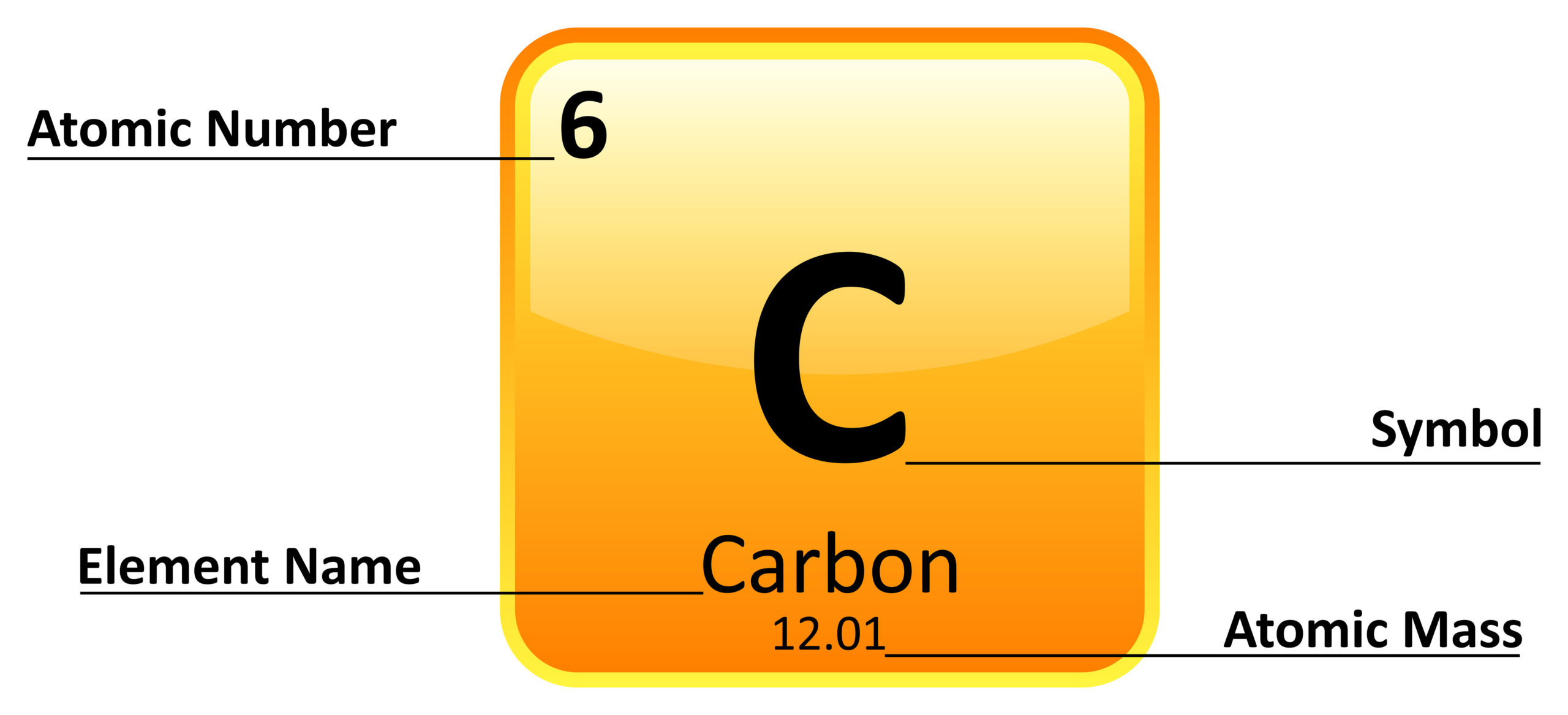
Element
A kind of atom / a substance made entirely from one kind of atom, e.g., silver, or hydrogen.
8
New cards
Compound
Any substance containing two or more kinds of atom chemically bonded together, e.g water and salt.

9
New cards
Molecule
The representative particle for molecular compounds and diatomic elements (e.g., O2, H2 and Cl2) is the ________.
10
New cards
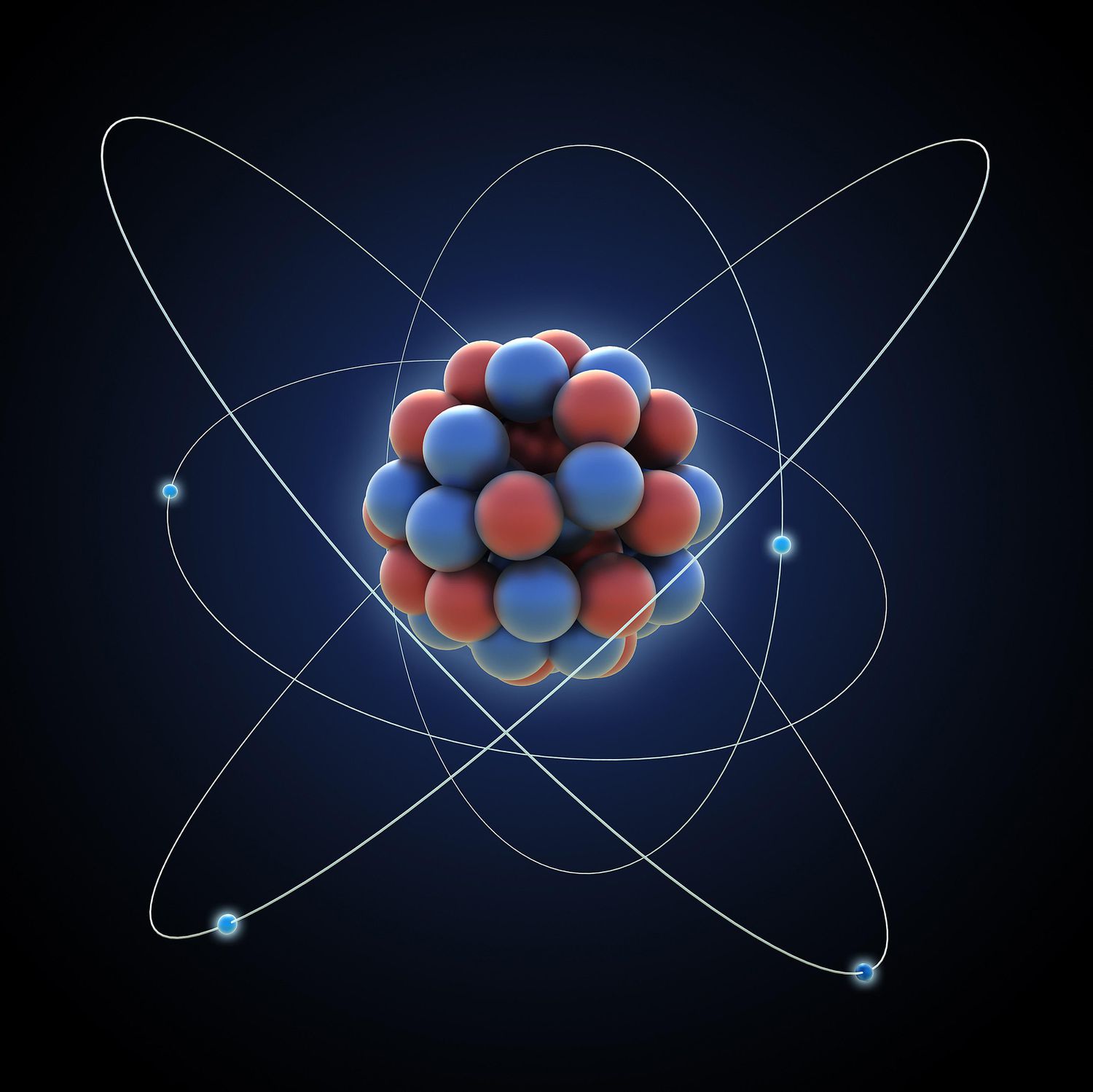
Atom
The building blocks of matter, made of protons, neutrons and electrons.
11
New cards
6\.02x10^23
Avogadro’s number
12
New cards
N(A)
The symbol of Avogadro’s number
13
New cards
x^(5+2)
Multiply the coefficient
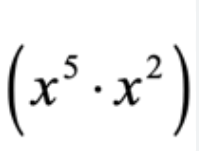
14
New cards
x^(6-5).y^(7-3)
Divide the coefficient

15
New cards
m = n \* M
Calculating the mass of a substance
16
New cards
M = n \* m
Calculating the molar mass of a substance
17
New cards
n = m / M
Calculating the number of mole of a substance
18
New cards
V = n \* 22.4
Calculating the volume of a gas
19
New cards
n = V / 22.4
Calculating the number of mole of a gas
20
New cards
Avogadro’s hypothesis
Amedeo Avogadro suggested that equal volumes of gas, at the same temperture and pressure, contain the same number of particles. In other words, the volume of 1 mole of any gas is always the same.
21
New cards
22\.4
The molar volume of a gas
22
New cards
n = N / 6.02x10^23
The calculation of number of moles of the representative particles
23
New cards
N = n \* 6.02x10^23
The calculation of the number of representative particles
24
New cards
N \* total number of atom
The calculation of the total number of atom
25
New cards
(mass of one element in one mole of compound / mass of compound) \* 100%
The calculation of the percent composition of a substance