Quiz 2 (copy)
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
C) order of increasing atomic mass
In the periodic table, the elements are arranged in __________.
A) order of increasing metallic properties
B) order of increasing neutron content
C) order of increasing atomic mass
D) reverse alphabetical order Periodic Families
C. Atomic Mass
It measures mainly of the nuclear particles: protons + neutrons.
A) Subatomic Particles
B) Atomic Number
C) Atomic Mass
D) Gluons
C) C, Si
Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties?
A) H, Li
B) Cs, Sr
C) C, Si
D) Ga, Ge
E) C, O
B) Periodic Table
The arrangement of elements organized by atomic number.
A) Electron Cloud
B) Periodic Table
C) Electron Configuration
D) Groups
Vertical columns of the periodic table are known as __________.
A) Metals
B) Periods
C) Nonmetals
D) Groups
E) Metalloids
A) 123
If the atomic number of Pb (Lead) is 82 and its atomic mass is 205, how many neutrons does it have?
A) 123
B) 82
C) 205
D) 287
A) Periods
Horizontal rows of the periodic table are known as __________.
A) Periods
B) Groups
C) Metalloids
D) Metals
E) Nonmetals
C) 1 proton and 2 neutrons
Hydrogen has an atomic mass of 3 and atomic number of 1. How many protons and neutrons does hydrogen have?
A) 3 protons and 1 neutron
B) 2 protons and 1 neutron
C) 1 proton and 2 neutrons
D) 1 proton and 3 neutrons
C) 1s2 2s2 2p6 3s2 3p6 4s2 3d6
The ground state electron configuration of Fe is __________.
A) 1s2 2s2 3s2 3p6 3d6
B) 1s2 2s2 2p6 3s2 3p6 4s2
C) 1s2 2s2 2p6 3s2 3p6 4s2 3d6
D) 1s2 2s2 2p6 3s2 3p6 4s2 4d6
B) Ar
Which element is the heaviest?
A) Na
B) Ar
C) Ca
D) O
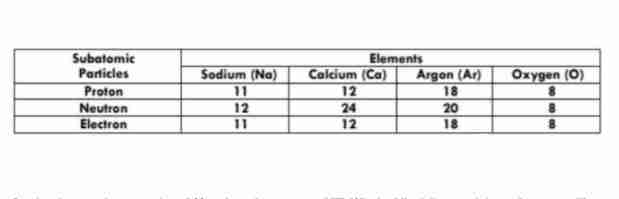
A) p 35, n 45, e 35
How many numbers of protons, neutrons, and electrons does Bromine-80 have?
A) p 35, n 45, e 35
B) p 35, n 35, e 40
C) p 40, n 40, e 35
D) p 40, n 35, e 40
D) O
Which atom has an atomic mass of 16? Use the table in number 6.
A) Na
B) Ar
C) Ca
D) O
D) Hund’s Rule
The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________
A) Pauli Exclusion Principle
B) Aufbau Principle
c) Octet Rule
D) Hund's rule
B) It has 11 protons and 11 electrons
An atom has an atomic number of 11 and an atomic mass of 23. Which of the following statements is correct?
A) It has 11 protons and 11 neutrons.
B) It has 11 protons and 11 electrons.
C) It has 11 electrons and 12 protons.
D) It has 23 protons and 11 electrons.
A) 6
The p-orbital can accommodate a maximum of __________ electrons.
A) 6
B) 2
C) 10
D) 3
E) 5
C) SrCl2
Which of the following compounds would you expect to be ionic?
A) H2O
B) CO2
C) SrCl2
D) SO2
A) 1-
Fluorine forms an ion with a charge of __________.
A) 1-
B) 1+
C) 2+
D) 3+
E) 3
A) barium, chlorine
Which pair of elements is most likely to form an ionic compound with each other?
A) barium, chlorine
B) calcium, sodium
C) oxygen, fluorine
D) sulfur, carbon
A) PCl5
Of the choices below, which one is not an ionic compound?
A) PCl5
B) CrCl6
C) RbCl
D) PbCl2
E) NaCl
C) Chalcogens
What group in the periodic table would the fictitious element :X: be found?
A) Alkaline Earth metals
B) Halogens
C) Chalcogens
D) Alkali metals
C) ionic bond
What type of bond forms between Be & F?
A) no bond forms
B) covalent bond
C) ionic bond
D) metallic bond
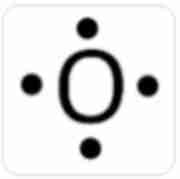
False
This is a correct dot diagram for oxygen (O)
A) True
B. False
A) lose electrons
What do atoms that form positive ions tend to do?
A) lose electrons
B) gain electrons
C) lose protons
D) gain protons
A) transfer of electrons
Ionic bonding involves
A) transfer of electrons
B) holding onto electrons
C) sharing of electrons
D) electrons are not involved
B) electron found in outermost shell
What is a valence electron?
A) electron found in a middle shell
B) electron found in outermost shell
C) electron found in innermost shell
D) electron not found in a shell
D) 2
Hydrogen being an exception to the octet rule, needs _____ electrons in its outer energy level to be stable.
A) 4
B) 6
C) 8
D) 2
B) nonmetals, negative, gain
Anions are _______ that form ____________ ions and __________ electrons.
A) nonmetals, positive, lose
B) nonmetals, negative, gain
C) metals, positive, gain
D) metals, positive, lose
B) Aufbau Principle
Electrons occupy orbitals of lowest energy first is part of what electron configuration rule?
A) Hund’s Rule
B) Aufbau Principle
C) Pauli Exclusion Principle
B) decrease; increase
The size of atoms ___ to the right and ___ as you go down the periodic table
A) increase; decrease
B) decrease; increase
C) decrease; decrease
A) representative elements
The Group A (1,2,13-17) elements of the periodic table.
A) representative elements
B) symbolic elements
C) compound elements
D) complex elements
A) Magnesium and Calcium
Which two elements will display the most similar chemical properties?
A) Magnesium and Calcium
B) Nickel and phosphorus
C) Sodium and oxygen
D) Aluminum and potassium
B) Hydrogen
Which element has an isotope that does not contain neutrons?
A) Helium
B) Hydrogen
C) Nitrogen
D) Neon
B) groups
It is classified into two family. Family A which is the representative elements and Family B which is the transition metals
A) blocks
B) groups
C) periods
D) orbitals
A) when they are arranged in order of increasing atomic mass
The periodic law states that there is periodic repetition of the physical and chemical properties of elements –
A) when they are arranged in order of increasing atomic mass.
B) if only metals are considered.
C) when they are arranged in order of increasing atomic radii.
D) when they are arranged in order of increasing atomic number.
B) FALSE
The higher the atomic number of an element, the bigger its atomic radius is.
A) TRUE
B) FALSE
B) FALSE
Elements with high valence electrons are likely to form cations.
A) TRUE
B) FALSE
D) Moseley
Who among these chemists arranged the elements in order of increasing atomic number?
A) Meyer
B) Mendeleev
c) Lavoisier
D) Moseley
B) Oxygen
Which of these elements will form an anion?
A) Potassium
B) Oxygen
C) Calcium
D) Boron
D) Tin
The following are metalloids EXCEPT for _________.
A) Antimony
B) Boron
C) Germanium
D) Tin
C) Group 7A
Which of these groups is known as the Halogens?
A) Group 1A
B) Group 2A
C) Group 7A
D) Group 8A
A) Metal, Nonmetal
Copper is a __________ and helium is a __________.
A) Metal, Nonmetal
B) Metal, Metal
C) Metalloid, Nonmetal
D) Nonmetal, Meta
A) B
Which of the following elements is a metalloid?
A) B
B) C
C) Ga
D) Se
C) Alkali Metals
Elements in Group 1 are known as the __________.
A) Oxygen Family
B) Alkaline Earth Metals
C) Alkali Metals
D) Halogens
8
How many significant figures are there in 5.0000000?
Johann Wolfgang Döbereiner
Who proposed the Law of Triads?
cation
What do you call the atoms that donates their valence electrons to make other atoms stable during chemical bonding?
3380
Solve and give your answer with the correct number of significant figures. (12.470)(271)
36.8
Solve and give your answer with the correct number of significant figures 24.28 + 12.5
4.078 × 10^-3
Change from standard form to scientific notation: 0.004078