Myoglobin & Hemoglobin
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Myoglobin
Small intracellular protein in vertebrate muscles
The colour of myoglobin represents the colour of meat (red or purple)
Myoglobin binds O2, and its major function is to facilitate oxygen diffusion in muscles; it can also store oxygen.
Even though myoglobin facilitates the diffusion of oxygen, it is not actually essential under normal conditions.
Myoglobin content in marine life is much higher than in land animals.
Myoglobin structure
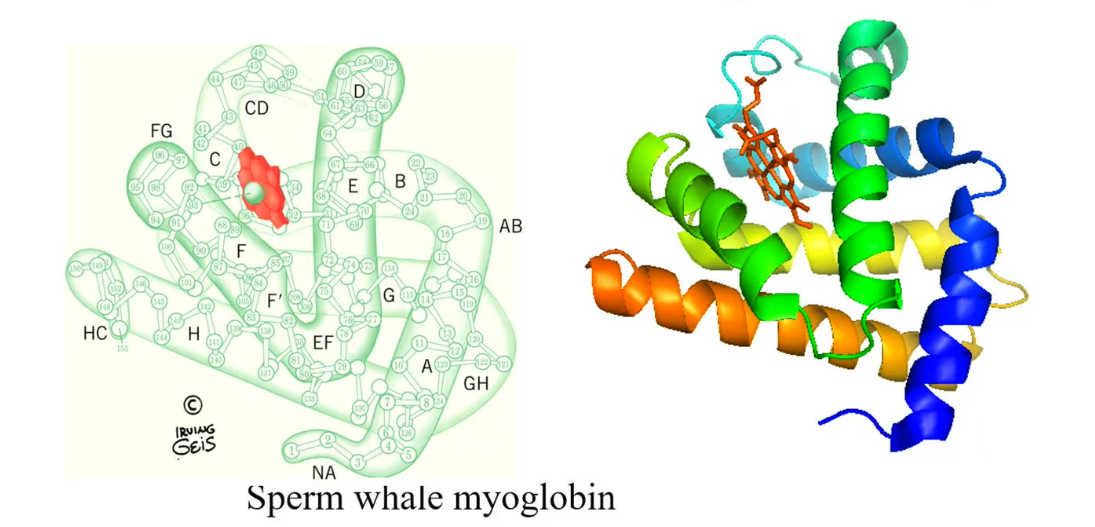
Contain only 153 amino acids, the first protein with a known x-ray structure.
The specific protein that was analyzed in this case was the sperm whale myoglobin.
Human myoglobin contains 154 amino acids
The protein structure has 8 helices: there named from A to H, which contain short inter helices region (junctions), such as CD, EF, FG and GH (shown in the diagram)
Myoglobin: Heme group
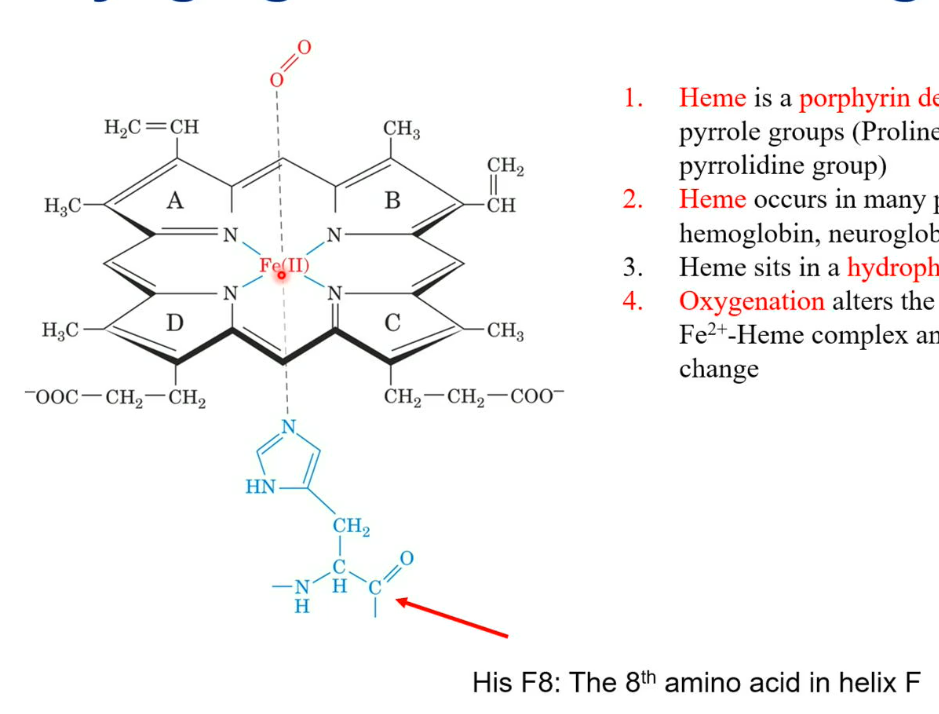
Heme is a porphyrine derivative containing four pyrrole groups (proline side chain is a pyrrolidine group). There is also a histidine side chain, which comes from the F helix (on the myoglobin) 8th amino acid. On the other side, there is oxygen, which binds to the iron.
Heme occurs in many proteins: myoglobin, hemoglobin, neuroglobin, cytochrome, etc.
Oxygenation alters the electronic state of the Fe2+- Heme complex and causes the colour change (purple to red)
Myoglobin molecule visualization with oxygen in heme group
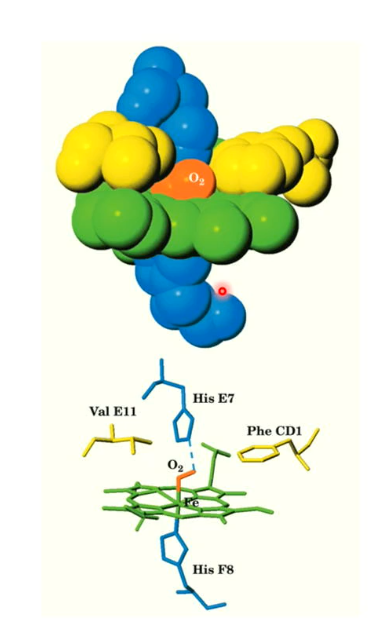
Here we see the oxygen is attached to the heme group, and His E7 attaches with the O2 and myoglobin’s structure tightly surrounds the heme group and the oxygen.
The Val E11 and Phe CD1 help hold the heme in place
The oxygen is known as oxygenation because the oxygen is not covalently bonded to the heme group; it can be easily released.
Dissociation constant for myoglobin
Because the reaction Mb + O2 ←→ MbO2 is reversible, there can be a equilibrium constant, known as k.
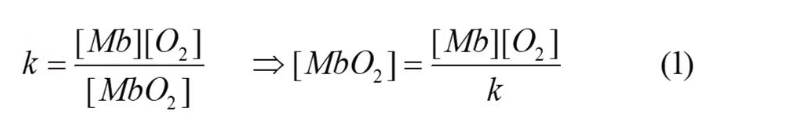
Fractional Saturation
This is the fraction of O2 - O2-binding site occupied by O2, pretty much, how many myoglobin molecules actually have oxygen bonded to them.
There are two fractional saturation formulas
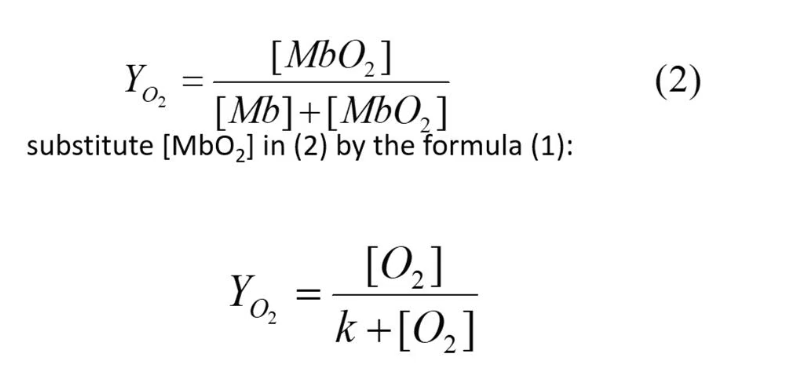
Fractional Saturation (partial pressure)
Oxygen is a gas, thus it is usually expressed as the partial pressure (oxygen tension) pO2
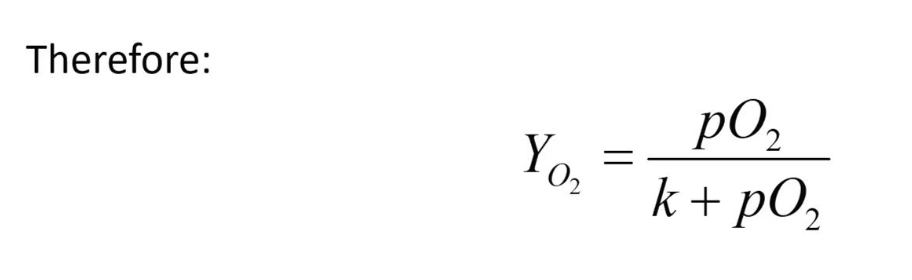
Oxygen bindings curve of myoglobin
As concentration of pO2 increases, the fractional saturation also increases, but it starts to slow down eventually. It slows down and will never reach 1, because the denominator has the constant k being added to pO2 thus the denominator will always be a larger value than the numerator.
When fractional saturation (Yo2) is 0.5 (50%) or 50% of myoglobin is saturated, the k value equals pO2 concentration
1 atm = 760 torr

Hemoglobin function
Found in our red blood cells
Structurally (homologue) related to myoglobin
But only 18% of residues are identical in myoglobin and in the alpha or beta subunits of hemoglobin
Functions for O2 transport
Hemoglobin quaternary structure
Hemoglobin is a tetramer
It has 4 monomers, 2 alpha and 2 beta
Has an exact C2 symmetry structure, but if we assume alpha and beta are the same, then it has a pseudo-D2 symmetry.
Has promoters, the alpha and beta subunits
In alpha subunit, there is no D helix
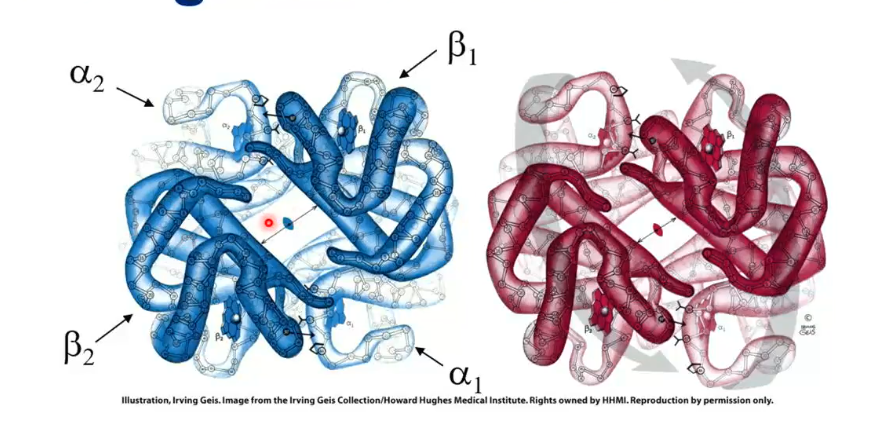
The binding of O2 causes a conformational change of the tetramer: the alpha-beta protomer rotates 15 degrees upon O2 binding and brings the beta chains closer
The blue structure in the picture is the hemoglobin with no oxygen attached, and the red is with oxygen attached.
Hemoglobin vs Myoglobin Oxygen Binding: Key Differences
Hemoglobin binds oxygen (O₂) in a sigmoidal (S-shaped) curve, which means at low oxygen pressure (pO₂), its binding is weak, but at high pO₂, binding gets much stronger.
The sigmoidal curve is a sign of cooperative binding—a feature where the binding of one oxygen molecule increases the likelihood that more oxygen will bind. This is unique for hemoglobin.
In contrast, myoglobin displays a hyperbolic curve, meaning its oxygen binding is independent (not cooperative): binding one oxygen does not affect binding at other sites.
At low pO₂ (such as in veins), hemoglobin binds oxygen less than myoglobin. At high pO₂ (such as in arteries), hemoglobin binds more oxygen, thanks to its cooperative mode.
Summary: Myoglobin is better at grabbing oxygen at low levels, but hemoglobin can drastically increase its oxygen binding as oxygen becomes more available—this helps it deliver oxygen efficiently throughout the body.
![<ul><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Hemoglobin</strong> binds oxygen (O₂) in a sigmoidal (S-shaped) curve, which means at low oxygen pressure (pO₂), its binding is weak, but at high pO₂, binding gets much stronger.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">The <strong>sigmoidal curve</strong> is a sign of cooperative binding—a feature where the binding of one oxygen molecule increases the likelihood that more oxygen will bind. This is unique for hemoglobin.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">In contrast, <strong>myoglobin</strong> displays a hyperbolic curve, meaning its oxygen binding is independent (not cooperative): binding one oxygen does not affect binding at other sites.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">At <strong>low pO₂</strong> (such as in veins), hemoglobin binds oxygen less than myoglobin. At <strong>high pO₂</strong> (such as in arteries), hemoglobin binds more oxygen, thanks to its cooperative mode.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Summary:</strong> Myoglobin is better at grabbing oxygen at low levels, but hemoglobin can drastically increase its oxygen binding as oxygen becomes more available—this helps it deliver oxygen efficiently throughout the body.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/e66dae77-5a18-46aa-b02f-654fc693758c.png)
How many heme groups in a molecule of hemoglobin?
It has 4 heme groups, thus can bind 4 oxygens.
As the hemoglobin binds more oxygen, its affinity towards binding more oxygen increases.
Hill Equation and Hemoglobin Cooperativity
The Hill equation was developed by Archibald Hill in 1910 to analyze how hemoglobin binds oxygen (O₂).
Hill assumed hemoglobin could bind multiple O₂ molecules all at once, with infinite cooperativity (all or none binding).
The Hill equation mathematically models the degree of hemoglobin oxygen saturation (YO2) based on oxygen pressure (pO2), using an exponent n for cooperativity: (attached)
In real life, hemoglobin does not bind all 4 O₂ molecules at once, and the actual cooperativity (n) can be a non-integer value, reflecting partial cooperative binding.
![<ul><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">The Hill equation was developed by Archibald Hill in 1910 to analyze how hemoglobin binds oxygen (O₂).</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">Hill assumed hemoglobin could bind multiple O₂ molecules all at once, with <em>infinite cooperativity</em> (all or none binding).</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">The Hill equation mathematically models the degree of hemoglobin oxygen saturation (<span style="font-family: KaTeX_Main, "Times New Roman", serif; line-height: 1.2; font-size: 1.21em;"><span>YO2</span></span>) based on oxygen pressure (<span style="font-family: KaTeX_Main, "Times New Roman", serif; line-height: 1.2; font-size: 1.21em;"><em><span>pO</span></em><span>2</span></span>), using an exponent <span style="font-family: KaTeX_Main, "Times New Roman", serif; line-height: 1.2; font-size: 1.21em;"><em><span>n</span></em></span> for cooperativity: (attached)</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><em>In real life,</em> hemoglobin does not bind all 4 O₂ molecules at once, and the actual cooperativity (n) can be a non-integer value, reflecting partial cooperative binding.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/feb93e12-83fa-41d8-9fcf-478b20f96243.png)
Hill Plot, Hemoglobin Affinity, and Oxygen Concentrations
The Hill equation and its logarithmic form are used to analyze hemoglobin’s oxygen binding cooperativity.
On the log-log plot, the slope for myoglobin is 1 (no cooperativity), while hemoglobin has a slope of about 3, showing cooperative binding.
The first oxygen binds to hemoglobin at a relatively high oxygen pressure (pO2pO2 ≈ 30 torr), but the last (4th) oxygen binds at a much lower pressure (pO2pO2 ≈ 0.3 torr).
Summary: At low oxygen concentrations, hemoglobin’s binding is weak, while at high oxygen concentrations it binds oxygen much more strongly (higher affinity). This change in affinity is due to cooperativity between hemoglobin subunits.
![<ul><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">The Hill equation and its logarithmic form are used to analyze hemoglobin’s oxygen binding cooperativity.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">On the log-log plot, the slope for <strong>myoglobin</strong> is 1 (no cooperativity), while <strong>hemoglobin</strong> has a slope of about 3, showing cooperative binding.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">The first oxygen binds to hemoglobin at a relatively high oxygen pressure (<span style="font-family: KaTeX_Main, "Times New Roman", serif; line-height: 1.2; font-size: 1.21em;"><span>pO2</span><em><span>pO</span></em><span>2</span></span> ≈ 30 torr), but the last (4th) oxygen binds at a much lower pressure (<span style="font-family: KaTeX_Main, "Times New Roman", serif; line-height: 1.2; font-size: 1.21em;"><span>pO2</span><em><span>pO</span></em><span>2</span></span> ≈ 0.3 torr).</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Summary:</strong> At <strong>low oxygen concentrations,</strong> hemoglobin’s binding is weak, while at <strong>high oxygen concentrations</strong> it binds oxygen much more strongly (higher affinity). This change in affinity is due to cooperativity between hemoglobin subunits.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/743fa167-2589-448b-b906-fd46372cb960.png)
Why does hemoglobin have the such a binding property (affinity-based)?
When oxygen (O₂) binds to hemoglobin, it causes the Fe²⁺ ion in the heme group to shift in position, dragging the attached histidine (His F8) by 0.6 Ångström.
This movement causes Helix F in the protein to tilt by about 1 Ångström, triggering a conformational change in the whole protein.
This change is what switches hemoglobin from its tight “T state” (deoxyhemoglobin) to its relaxed “R state” (oxyhemoglobin), enabling efficient oxygen transport.
![<ul><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">When oxygen (O₂) binds to hemoglobin, it causes the Fe²⁺ ion in the heme group to shift in position, dragging the attached histidine (His F8) by 0.6 Ångström.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">This movement causes Helix F in the protein to tilt by about 1 Ångström, triggering a <em>conformational change</em> in the whole protein.</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">This change is what switches hemoglobin from its tight “T state” (deoxyhemoglobin) to its relaxed “R state” (oxyhemoglobin), enabling efficient oxygen transport.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/33d1fbe8-97c3-46c9-a79e-17af888fa6fc.png)
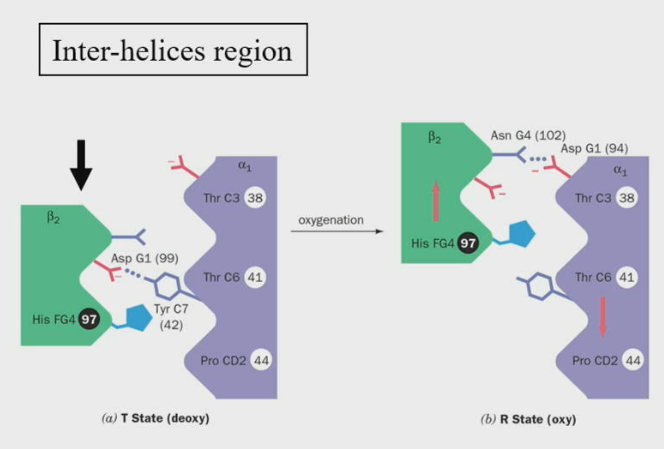
Changes at at the alpha1 - beta2 interface during the T —> R transition
The beta2 region shifts by one turn along the alpha1 C-helix
Notice the His FG4 side chain fits in the grooves of the alpha1 chain, before and after the transition.
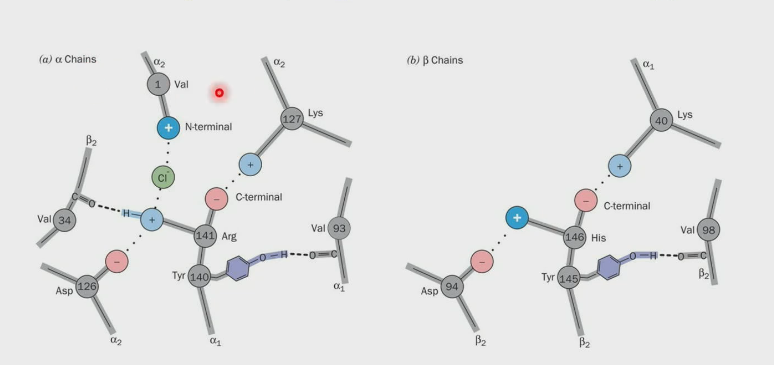
Disruption of ionic pairs due to T —> R transition in hemoglobin
Both the alpha and beta C-termini form ion pairs, these ion pairs are formed in the T state and the binding of O2 tears the ion pairs.
Bohr Effect
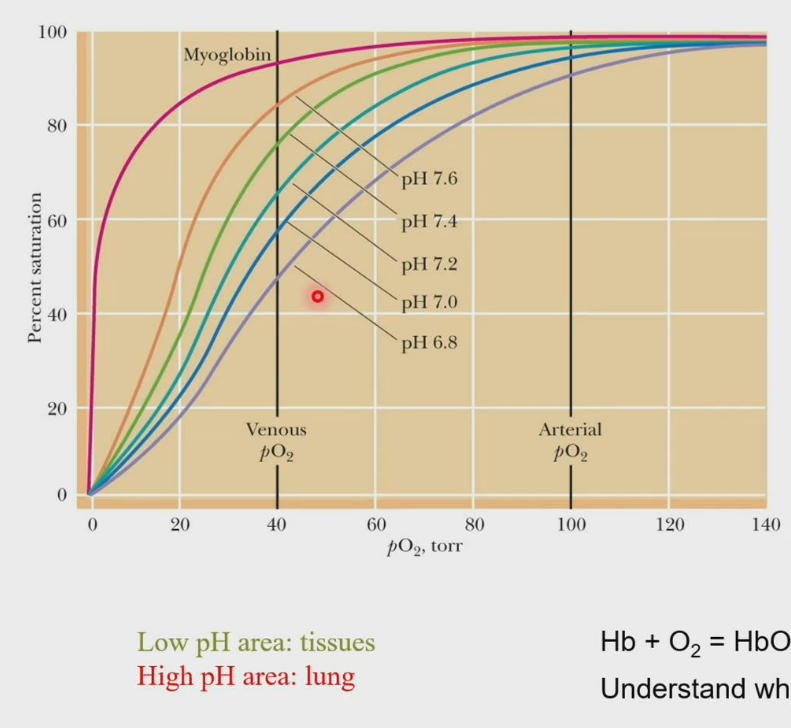
Disruption of ion pairs changes the pKa’s of the ionizable groups, and the overall consequence is: For every O2 binding, 0.6 proton is released. The pKa’s are decreased; therefore, the O2 affinity of hemoglobin increases with increasing pH.
Hb + O2 ←→ HbO2 + H+ (As hemoglobin binds oxygen, that process releases a proton).
In our lungs, pH is higher, thus it promotes hemoglobin to bind more oxygen, but in our tissues, the pH is lower, thus allowing for the release of oxygen in the tissues.
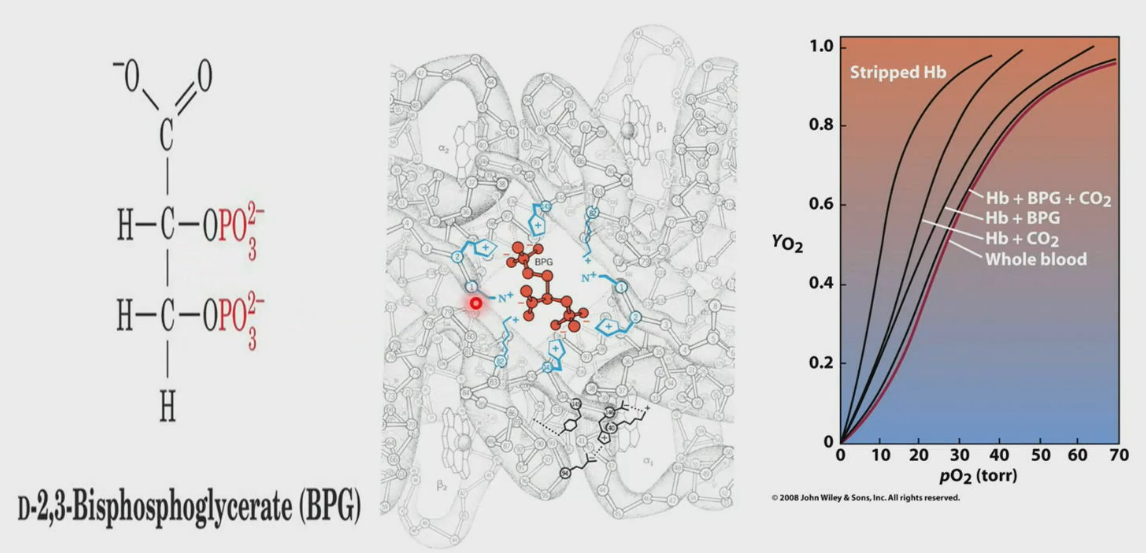
D-2, 3-Bisphosphoglycerate (BPG)
BPG occupies the channel of hemoglobin only in T-state. In R-state, the channel is much narrower. Binding of BPG helps with release of O2 in tissues.
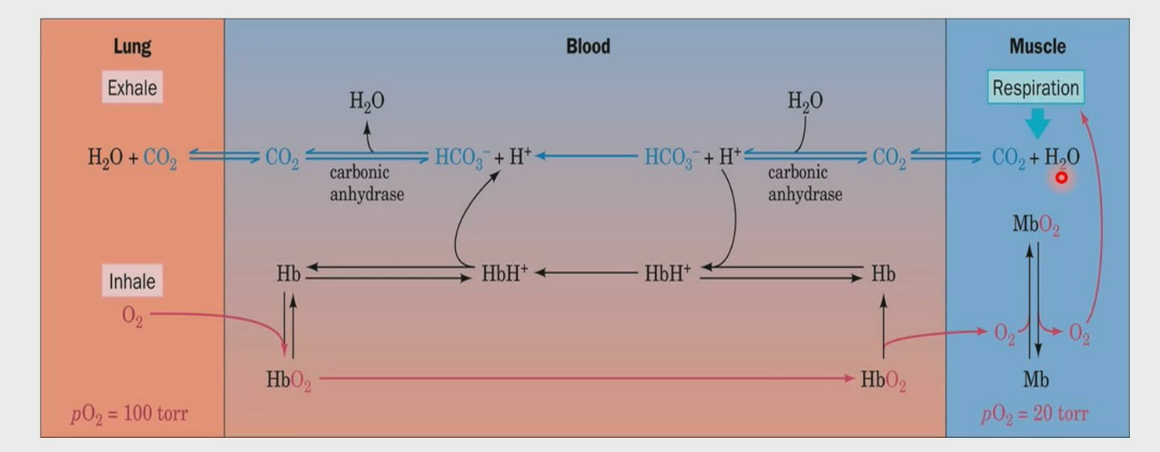
O2 and CO2 transport through the blood
Lungs have higher pH due to high pressure of oxygen, leading to uptake of oxygen by Hb. The Hb tan goes and transport oxygen to the tissue, in this case muscle. Mb uptakes the oxygen and uses it for respiration, where CO2 in gas form (not soluble) is released along with water. Carbonic anhydrase converts CO2 into HCO3-, which makes it soluble. It goes to the lungs, where carbonic anhydrase converts it back to CO2, and then you exhale it from your lungs.
CO2 can also bind to Hb directly, which forms carbamate and is taken to the lungs to be released.