2. Skin Delivery - Creams, ointments and other topical preparations
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What does topical mean in pharmacy?
Applied to external body surfaces for localized effect
Can include skin, scalp, eye, nasal, buccal, rectal & vaginal areas
In this context, refers only to skin & scalp (non-mucosal)
Why is treatment given locally to the skin?
Enhance skin’s barrier function
Target drug to specific skin layers (e.g. outer layer for fungal infections, deeper for eczema)
Fewer side effects vs oral drug delivery
What are the 3 main layers of the skin & key features?
Epidermis: outer layer; stratum corneum (outermost), stratum basale (where skin cells form); no blood vessels
Dermis: contains blood vessels, sweat glands, hair follicles, sebaceous glands
Subcutaneous: fatty tissue layer that cushions the skin
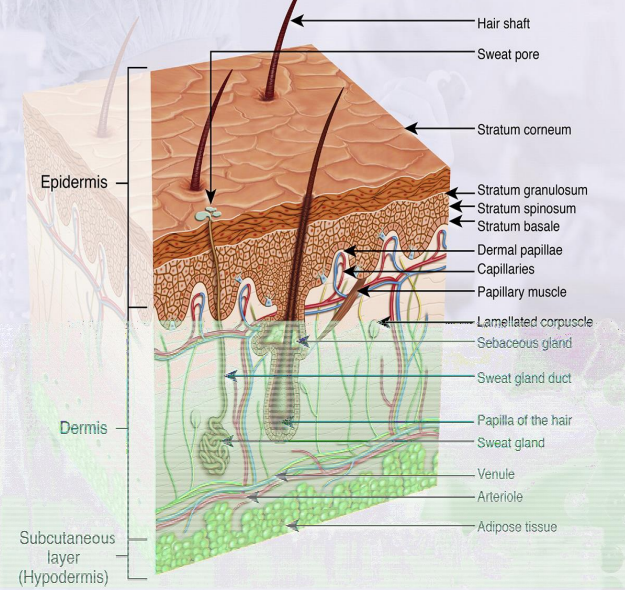
How does target location affect topical treatment?
Above/on SC: easier – e.g. sunscreens, insect repellents
Targeting SC: e.g. fungal infections
Below SC (epidermis/dermis): harder – requires drug to cross SC = percutaneous absorption
What types of topical dosage forms are available?
Semisolids (halfway between solid & liquid): ointments, pastes, creams, lotions, jellies, gels, liniments, collodions, plasters
Topical solutions, soaps/shampoos, tinctures, powders, aerosols/foams, topical patches
What is the difference between occlusive & non-occlusive topical formulations?
Occlusive: prevents water loss, ↑ skin hydration
Good for dry skin, not for infected skin
Forms a barrier/shield on skin
Enhances drug penetration through skin
What are ointments & what are they made of?
Semisolid preparations for external use on skin/mucosa
Made from hydrocarbons (not water-soluble)
Common ingredients:
Soft paraffin (main base)
Liquid paraffin (to thin)
Hard paraffin (to thicken)
What are the properties of hydrocarbon ointments?
Occlusive & emollient
↑ skin hydration by trapping moisture
Keeps skin supple
Greasy & hard to wash off with water
Useful if drug is unstable in water
What are water-soluble ointments?
Washable & water-soluble
Example: macrogol (PEG) ointment
Longer chain length = higher melting point
Used in:
Bactroban (mupirocin) for skin infections
Iodosorb (iodine) for wound care
What are the properties of water-soluble ointments?
Water soluble & washable
Non-greasy
Non-occlusive or less occlusive than hydrocarbon ointments
Do not lock in moisture as effectively as hydrocarbon bases
What are creams & why are they popular for topical use?
Two phases: oil & water (O/W or W/O)
O/W more common for drug delivery
Need emulsifiers to keep stable & preservatives due to water content
More popular because:
Softer, less sticky than ointments
Spread easily, good patient acceptability
O/W creams leave a thin film after water evaporates
Less occlusive than ointments (don’t lock in moisture as well)
What are examples of W/O emulsifiers and creams?
Wool alcohols (lanolin alcohols): Key W/O emulsifier, rich in cholesterol & lanesterol
Hydrous wool fat (hydrous lanolin): 7 parts wool fat, 3 parts water, softer than wool fat
Beeswax: Traditional W/O emulsifier
Examples of W/O creams:
Drapolene Cream (for nappy rash)
Boots Chilblain Cream
What are examples of O/W emulsifiers and creams?
Emulsifying Waxes (O/W):
Emulsifying wax BP: Sodium lauryl sulphate & cetostearyl alcohol (1:9)
Cetrimide emulsifying wax BPC: Cetrimide & cetostearyl alcohol (1:9)
Cetomacrogol emulsifying wax BPC: Cetomacrogol 1000 & cetostearyl alcohol (8:2)
Examples of O/W creams:
Aqueous Cream (O/W): Emulsifying ointment (O), water (W), preservative
Hydrocortisone cream (O/W): Mineral oil (O), water (W), emulsifiers, co-solvents, preservative
What are the properties & an example of topical lotions?
Properties:
Liquid with insoluble solids that need suspension
Low viscosity → spreads easily
Dries quickly on skin after application
Example: Calamine lotion
Contains calamine, zinc oxide (solids)
Glycerol (moisturises), phenol (antibacterial)
Bentonite (keeps solids suspended), water
Pink colour from iron oxide in calamine
What are gels & how do gelling agents work?
Gels:
Solid or semisolid preparations
Usually transparent or translucent
Require gelling agents (natural: tragacanth, pectin, alginate; synthetic: carbomer, cellulose)
E.g. Ibugel, isotrexin gel
Gelling agents:
Form a cross-linked network in liquid
Gels = liquid dispersed in solid (solid is continuous phase)
Hydrogels form when hydrophilic polymers swell in water
What are carbomer gels & how are they formed?
Carboxy vinyl polymers
Form low viscosity dispersions in acidic water
Gel forms when neutralized with alkali
How does the target site affect the choice of topical formulation?
Sunscreens → stay on surface → use gels, sprays, creams, lotions (not ointments)
Fungal infections → affect stratum corneum → target outer skin layers (not ointments)
Inflamed skin → barrier issues → ointments useful (occlusive & protective)
How is transdermal delivery different from topical delivery?
Topical → local effect, no bloodstream entry
Transdermal → drug enters bloodstream
Avoids breakdown in stomach/gut
↑ patient compliance