Desensitization of GPCRs
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
Desensitization of GPCRs
Important process where cells decrease their sensitivity to a NT to prevent saturation of the system. Defined: an increase, in the concentration of NT required to produce half-maximal stimulation of the effector enzyme (for example). Less of a response for a constant amount of NT.
Two main modes of desensitization
Phosphorylation: rapid and receptor mediated endocytosis: slow
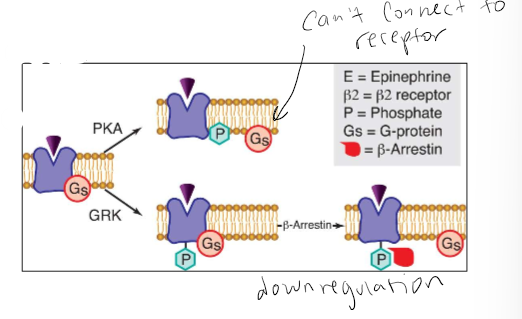
Phosphorylation
Rapid. Phosphorylation of intracellular loop of the GPCR: stops binding of G-protein. PKA: protein kinase A. GRK: G-protein receptor kinasel which then recruits B-arrestin. This could lead to receptor mediated endocytosis.
Receptor mediated endocytosis
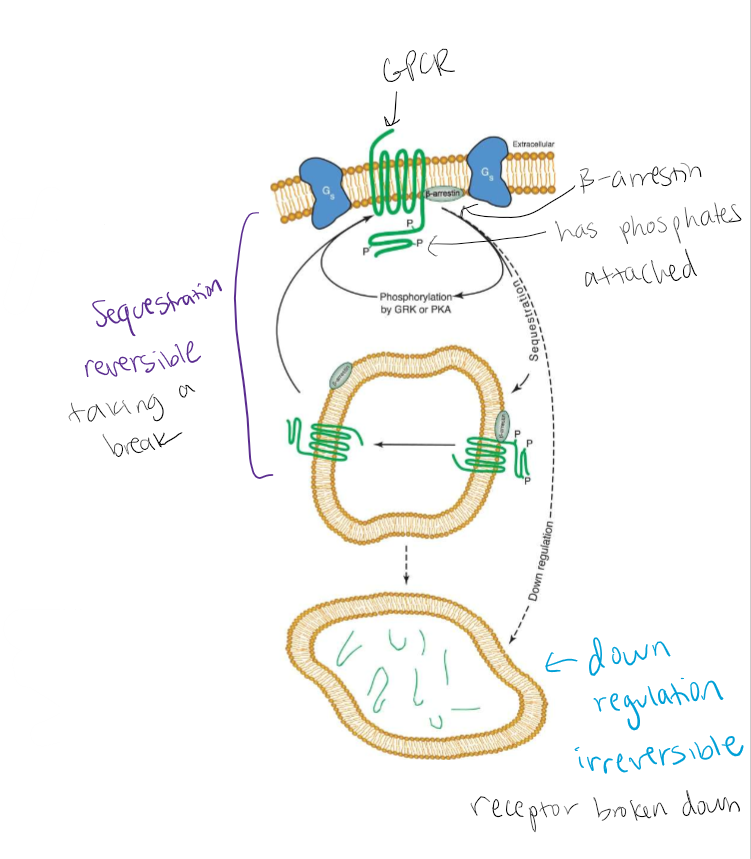
Slow. Reversible: sequestration. Irreversible: down-regulation (receptor degradation)

GRK Phosphorylation and B-arrestin Recruitment greatly enhances endocytosis. All have similar properties
Sequestration vs down regulation
Figure 10.20 Additional intracellular pathways associated with desensitization of GPCRs. GPCRs are phosphorylated (noted with P) on their intracellular domains by PKA, GRK, and other protein kinases. The phosphorylated form of the receptor can be removed from the cell surface by a process called sequestration with the help of the adapter protein β-arrestin; thus, fewer binding sites remain on the cell surface for transmitter interactions. In intracellular compartments, the receptor can be dephosphorylated and returned to the plasma membrane in its basal state. Alternatively, the phosphorylated receptors can be degraded (downregulated) by targeting to a lysosomal organelle. Degradation requires replenishment of the receptor pool through new protein synthesis.