2.1.2 Biological Molecules
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
What is the structure of water/ How is it bonded
2 hydrogen atoms covalently bonded to 1 oxygen atom
Hydrogen shares a pair of electrons with oxygen. The oxygen has a greater affinity for the electrons (because it’s larger) so it pulls the electrons (and consequently the hydrogen) closer
Oxygen is slightly delta negative and hydrogen is slightly delta positively charged
This creates different charged regions which makes water a polar molecule. Because it has 2 it’s dipolar.
Water has a very stable structure due to its many hydrogen bonds, despite them being weak and despite water being a small molecule
Draw a water molecule
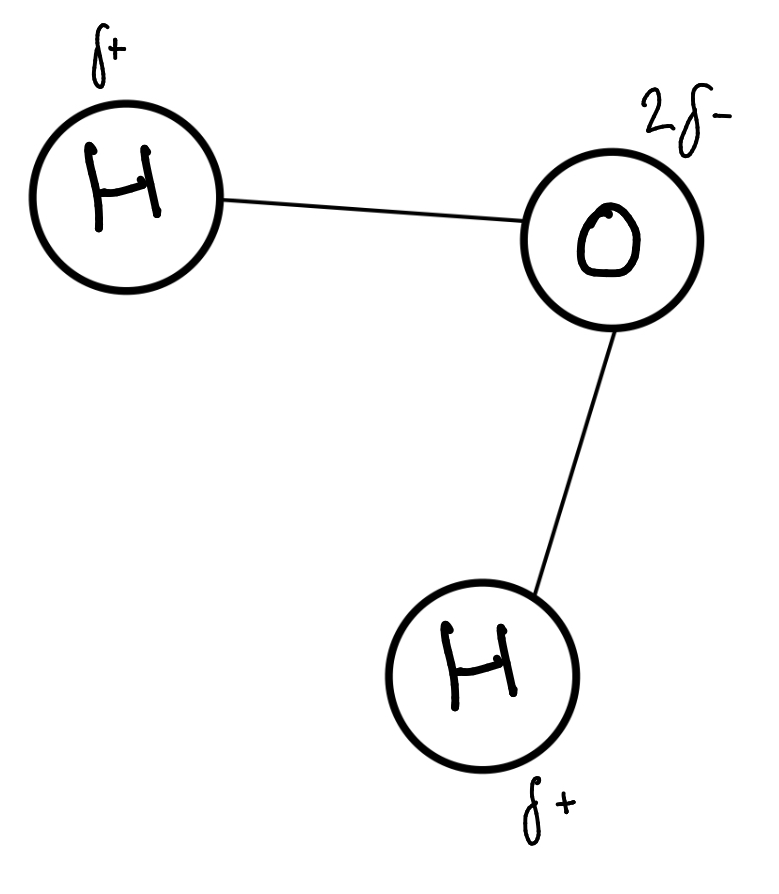
What gives water its high SHC and how is it used
This is due to water having many hydrogen bonds which require lots of energy to overcome
This means water is good at retaining heat and resists changes in temperature
Which allows organisms to live in them despite the climate
This also allows it to work as a coolant/moderator of temperature changes
What is cohesion in water
Cohesion allows water to move in one mass by allowing water molecules to stick together in chains
What is adhesion in water
Adhesion allows water to stick to other materials
Plants use this during transpiration when water moves up xylem vessels
Animals use this when transporting dissolved compounds around the body via capillary action
What gives water its surface tension and how is it used
Water molecules are more strongly cohesive to each other that to air, this results in water having a skin of surface tension
This therefore allows a habitat to exist on the surface of water for organisms like pond skaters
How does waters role as a solvent work
Many solutes of an organisms can be dissolved in water
It can dissolve most organic and inorganic substances
It is needed for all bio-chemical reactions
In animals: used to remove excretory products such as urea and excess salts
In plants: Root hairs absorb mineral salts present in soil in solution form
How does waters role as a insulator work
When water freezes into ice it forms an open lattice structure, which leads to more space between molecules causing them to expand
The expansion causes the density to decrease
The decrease in density causes it to float
In which it will form an insulating layer above the pond of water allowing organisms to survive under it
How does water work as a transport medium
It’s solvent properties and cohesion forces allows water to be a transport medium
In humans: Human blood plasma consists of 90% water
In plants: Sugar and mineral salts are transported in solution in vascular bundles
How does water work as a reagent
It is used in hydrolysis and condensation
What is a condensation reaction
A reaction where molecules are joined together by forming a covalent bond and removing water
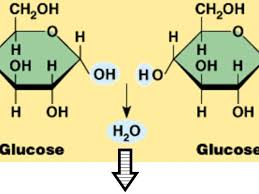
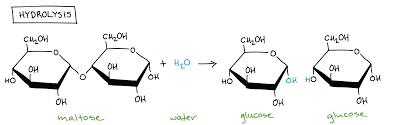
What is a hydrolysis reaction
A reaction that breaks a bond via the addition of a molecule of water
What are carbohydrates made up of
ONLY: Carbon, hydrogen, oxygen
What is Carbohydrates general formula
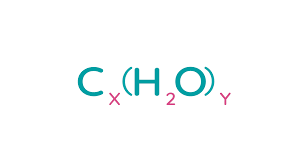
How do saccharides/sugars build up
Monosaccharides: 1 sugar, simple sugar
Disaccharides: Double sugars, formed from 2 monosaccharides
Polysaccharides: Large molecules formed from many monosaccharides
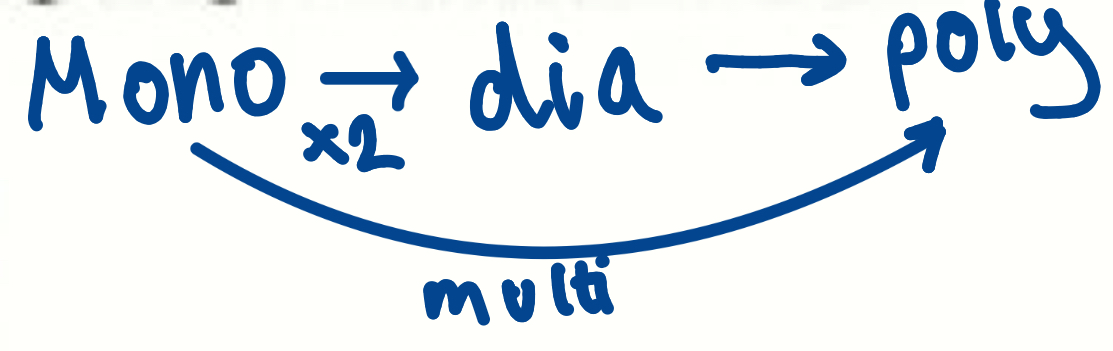
Examples of Monosaccharides (4)
Glucose
Fructose
Galactose
Ribose
Examples of Disaccharides (3)
Sucrose
Maltose
Lactose
Examples of polysaccharides (3)
Glycogen
Cellulose
Starch
What is a monosaccharide
Simplest ‘single sugars’
Same number of ‘C’ as ‘O’ atoms e.g. glucose is C6H12O6
If N=3 - triose e.g. glyceraldehyde
If N = 5 - pentose e.g. deoxyribose, ribose
If N = 6 - hexose e.g. glucose, galactose, fructose
White crystalline solids
They dissolve in water to form sweet tasting solutions
What is a hexose-glucose
Abundant and very important monosaccharide
It is a hexose sugar due to its 6 carbon atoms with a general formula of C6-H12-06
Major energy source for most cells
Highly soluble in water
Is the main form in which carbohydrates are transported around the body
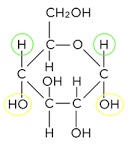
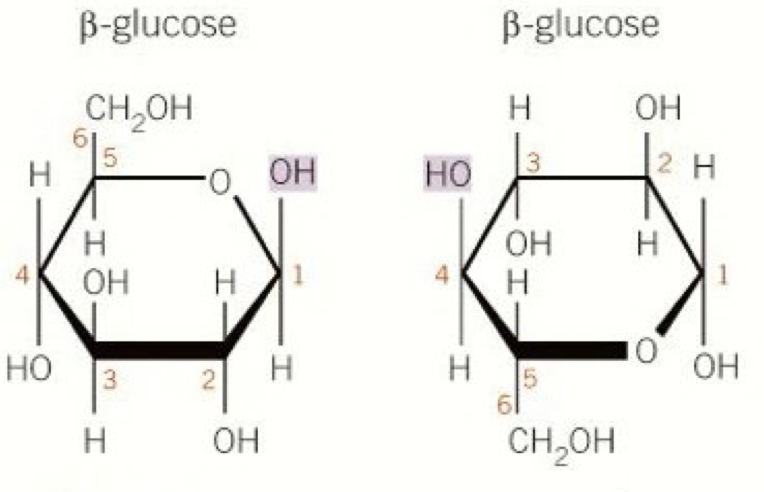
What are the different structural isomers of glucose called
Alpha (OH group below)
Beta (OH group above)
Draw the structure of (alpha) glucose
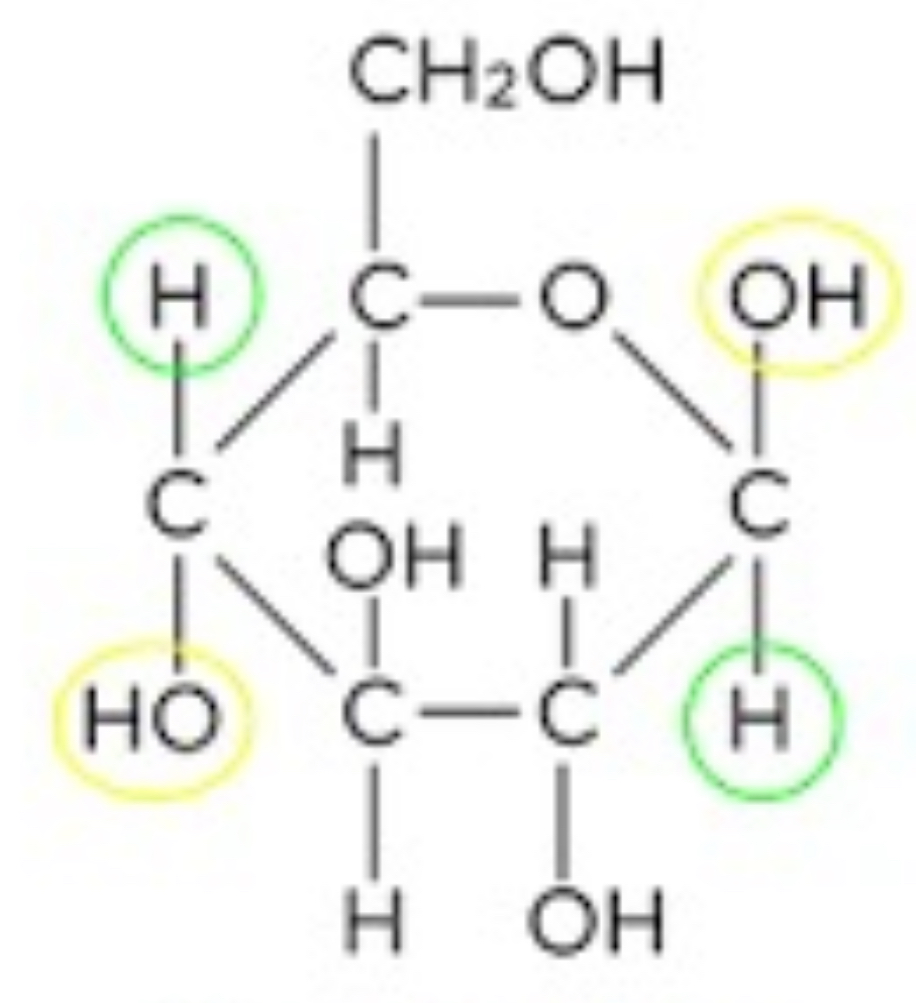
Draw the structure of beta glucose
How do monosaccharides bond to form disaccharides
The hydroxyl groups of the 2 glucose interact
The bond via a condensation reaction
One glucose bonds with its Carbon 1 and the other with its Carbon 4 to form a 1-4 glycosidic bond
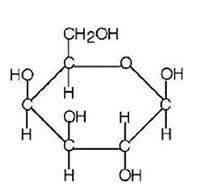
Draw the structure of galactose
Draw the structure of fructose
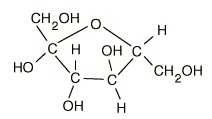
What are the features of fructose
Very soluble
Main sugar in fruits and nectar
Sweeter than glucose
What are the feature of galactose
Not as soluble as glucose
Has an important role in the production of glycolipids and glycoproteins
What is a pentose monosaccharide
They contain 5 carbon atoms
Examples include the structural isomers ribose and deoxyribose
Draw the structure of ribose
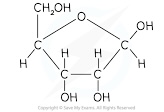
Draw the structure of deoxyribose
How is maltose formed
Formed from 2 glucose molecules
Joined by alpha 1-4 glycosidic bond
What is sucrose and how is it formed
Table sugar
Formed from 1 glucose and one fructose
Joined by an alpha 1-2 glycosidic bond
Fructose flips to allow this to happen
What is lactose and how is it formed
Milk sugars
Formed from 1 galactose and 1 glucose
Joined by a beta 1-4 glycosidic bond
How do you test for reducing sugars (All the monosaccharides)
The Benedict’s test
Benedict’s reagent is a turquoise liquid containing copper (II) sulfate ions in an alkaline solution
Why don’t reducing sugars don’t react with Benedict’s
The part of the molecules that needs to react is already in a glycosidic bond
How do you test for non-reducing sugars
Therefore they need to be hydrolysed before being tested
This is done by boiling with acid, neutralising and then boiling with Benedict’s
What is the structure of starch
20% of it is amylose in an alpha helix
Remaining 80% is amylopectin which is branched in a 1-6 glycosidic bond
Why do plants store glucose as starch
To get around the fact that glucose is soluble in water due to the hydroxyl groups
They can form hydrogen bonds with water molecules
What is the structure of amylose
Amylose is a polysaccharide of alpha glucose molecules In a 1-4 glycosidic bond
This then twists into an alpha helix - witch hydrogen bonds forming along the chain
What is the structure of Amylopectin
Highly branched
Can be hydrolysed quicker than amylose
due to being branched
gives it many ends
Many sites to break down starch to glucose
A-glucose molecules joined by 1-4 glycosidic bonds with a 1-6 bond every 20-30 monomers
Plants store it then hydrolyse it when they need a supply of energy
What are the properties and uses of starch
Major carbohydrate storage molecule in plants
Usually stored as intracellular grains in organelles called plastids
Includes green chloroplasts and colourless amylose
Produced from glucose made during photosynthesis
Broken during respiration to provide energy and is also a source of carbon
Compact due to double helix - can store lots of it
Insoluble - doesn’t cause osmosis for the cell its in
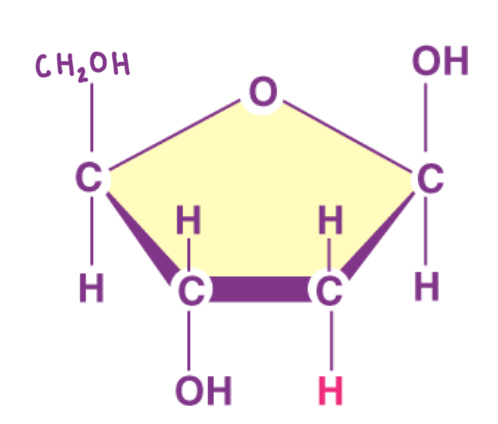
What is the structure of cellulose
Polysaccharide
Consists of long chains of beta glucose molecules joined by beta 1-4 glycosidic bonds
It is a straight chain molecule because it is unable to coil or form branches
This is due to the fact that each alternate molecule flips in order to have the hydroxyl group of the glucoses close enough to react
What are the features and roles of cellulose
Main component in cell walls
Most abundant organic polymer
Very strong - prevents cells from bursting when they take in water
Permeable
Humans cant digest cellulose as we dont produce cellulase (but cows do)
What is the structure of glycogen
Contains many alpha 1-6 glycosidic bonds that produce a very branched structure (similar to amylopectin)
What are the features and roles of glycogen
Animals store glucose as glycogen
Stored as small granules, particularly in the liver
Less dense ad more soluble than starch and is broken down quicker
Indicates the higher metabolic requirements of animals compared to plants
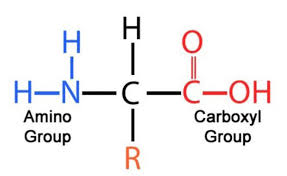
Draw the general structure of a protein
What are the different types of proteins
Structural: Proteins are the main components of body tissue - e.g. muscle, skin, ligaments and hair
Catalytic: All enzymes are proteins, catalysing many biochemical reactions
Signalling: Many hormones and all cell membrane receptors are receptors
Immunological: All antibodies are made up f proteins
What elements do all proteins contain
Carbon
Hydrogen
Oxygen
Nitrogen
What is the R group in a protein
It represents a side chain from the central ‘alpha’ carbon atom
It defines the amino acid - they all have the same general structure, the only different being the nature of the functional/R group
How many different types of amino acids that are used to make proteins are there
20
5 non-essential (Can be made from other amino acids)
6 conditionally essential (only needed for infants and growing children)
9 Essential (Can only be obtained by our diet)
How are polypeptides formed
Amino acids are joined together via condensation reactions to form a peptide bond making a dipeptide molecule
(dipeptide molecules are a transition between poly and peptides)
Dipeptides then have more amino acids added to them to create a polypeptide chain
The reaction is catalysed by the enzyme peptidyl transferase
A protein consists of one or more polypeptide chain folded into a highly specific 3D chain
What does the primary structure of a protein consist of
This is the sequence of amino acids within a polypeptide chain
The particular amino acids will influence how the polypeptide folds - this in turn determines its function
The only bonds involved in the primary structure are peptide bonds
What does the secondary structure of a protein consist of
Often the sequence of amino acids causes the polypeptide chain peptide to fold into a simple repeating pattern
Constitutes secondary structures such as alpha helixes and beta pleated sheets
The are held together by hydrogen bonds (non-covalent and weak) between the CO and NH groups of the chains
The O in the CO groups have a small negative charge
The H in the NH groups have a small positive charge
These charges can attract each other which can abuse hydrogen bonds to form which causes the chain to twist and fold
How is an Alpha helix formed
The shape is formed of 36 amino acids per 10 turns of the helix
Hydrogen bonds are formed between the CO of the carboxyl group and the NH of amine group of the amino acid 4 places ahead of it
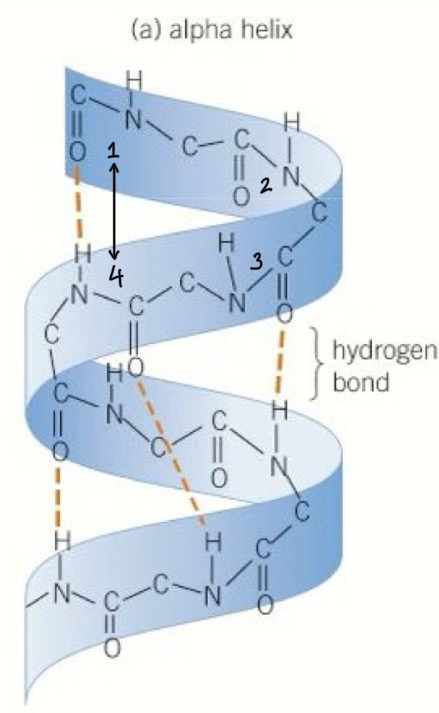
How is a beta pleated sheet formed
Polypeptide chains can also lie parallel to one another joined by hydrogen bonds, forming sheet like structures
What does the tertiary structure of a protein consist of
Often Includes sections of the secondary structure
The coiling and folding brings R-groups closer together so that they will interact and cause further folding
Interactions include:
Hydrophobic & Hydrophilic interactions: Weak interactions between polar and non-polar R groups (hydrophobic)
Hydrogen bonds: The weakest of the bonds formed, involved in all levels of structure, between polar R groups
Disulphide bonds/bridges: Covalent, one of the strongest, most important type of bond, occurs between R groups that contain sulphur atoms e.g. cysteine amino acids
Ionic bonds: Stronger than hydrogen bonds, forming between oppositely charged R groups
Van der Waals forces: between non-polar molecules
What does the quaternary structure of a protein consist of
Exists in proteins that have more than one polypeptide chain working together
Each polypeptide chain in the quaternary structure is referred to as a subunit of the protein
Interactions between the subunits are the same as in the tertiary structure but they are between different protein molecules rather then within one
Haemoglobin has 4 subunits, made up of 2 sets of 2 identical subunits
What is a globular protein
Compact
Spherical
Easily soluble (in water) - so they can be easily transported in fluids
How are globular proteins structured
Spherical shape caused by tightly folded polypeptide chains
Chains usually folded so that hydrophobic groups are on the inside, while hydrophilic ones are on the outside
this is what makes them soluble
What are conjugated proteins
A protein with a prosthetic group, that is non-protein
Part of the protein which enables it to do its job
Can be lipoproteins, glycoproteins, metal ions or minerals from vitamins
Attached to the main group via covalent bonds, ionic interactions and hydrogen bonds
E.g haemoglobin has a prosthetic haem group
What are the key inorganic cations that could be part of the prosthetic group
Calcium ions
Sodium ions
Potassium ions
Hydrogen ions
Ammonium ions
What are the key inorganic anions that could be part of the prosthetic group
Nitrate
Hydrogen
Carbonate
Chloride
Phosphate
Hydroxide
What is haemoglobin’s structure
Globular protein
Quaternary structure - four polypeptide chains (2 a-globin + 2 b-globin proteins) - each with a prosthetic haem group
Chains held together by disulphide bonds
Hydrophobic groups face inwards while hydrophilic ones face outwards
The haem group contains an Iron II ion which can reversible combine with oxygen to form oxyhaemoglobin
What are enzymes
Biological catalysts - they speed up the rate of reaction
Globular protein
E.g. Catalase
Converts excess hydrogen peroxide from metabolic reactions into water and oxygen, preventing any damage to cells or tissues
What is the structure of insulin
Globular protein
Has two polypeptide chains
A has 21 amino acid residues
B has 30 amino acids residues
2 chains held together by three disulphide bridges
What are fibrous proteins
High tensile strength (tough)
Long strands of polypeptide chais
Tend to be found in connective tissues e.g. tendons
Have little or no tertiary structure
Insoluble in water - due to large number of hydrophobic R groups
limited number of amino acids + R groups
The highly repetitive sequence creates very organised structures
Give and describe 3 examples of fibrous proteins
Keratin
Makes up hair, nails, horns and feathers-very tough
Rich in cytosine
Elastin
Found of in connective tissue, tendons, skin and bone
Can stretch and then return to its original form
Collagen
Connective tissue found in skin, tendions and ligaments
What is a lipid
Insoluble in water
Soluble in organic substances
Contain carbon, hydrogen + oxygen
But a higher proportion of hydrogen + a lower proportion of oxygen
Twice the energy per gram than carbohydrates
Used for long term storage in animals
Triglycerides most common type
What is the structure of triglyceride
Made up of 1 glycerol with 3 fatty acids
Glycerol = alcohol
Fatty acids = carboxylic acids
Both of them contain hydroxyl groups
They interact causing the formation of 3 water molecules and ester bonds
The reaction is caused esterfication - another example of a condensation
What are saturated fats
When a FA chain has the maximum number of hydrogen atoms (no more hydrogen can be added)
Long and straight chains
No carbon to carbon double bonds
Considered less healthy - implicated n elevated cholesterol synthesis
Generally solid
More abundant in fats of animal origin
What are unsaturated fats
Have at least one carbon to carbon double bond
Healthier (except trans fat)
Generally liquid at room temp
Found in oils that originate from plants
Fewer hydrogens due to carbon atoms in their chains
Monounsaturated - One carbon to carbon double bonds in each fatty acid chain while polyunsaturated have many
Double bond causes a kink in the hydrocarbon chain
Prevent unsaturated hydrocarbon chains packing closely together
Triglycerides containing a high proportion of unsaturated fatty acids have low melting temperatures
What are the different lipid functions (7)
Energy source
Energy store
Waterproof coverings - outer surface of insects and plants
Insulation against heat loss - under the skin of mammals
Electrical insulation - Fatty sheath around nerve cells - allows electrical impulses to travel faster
Protection of organs - Protects them from damage by absorbing shocks
Buoyancy - body fat of animals helps the, to float easily due to low density of fats
How do lipids work as an energy store
Oils are used as a fat store in plants
Seeds, e.g. sunflower
Fruits, e.g. palm, olive
Spare food often converted to fats or oils for use later
Animals short fat in adipose tissue (this surrounds many organs ad works as in insulator)
How do lipids work as a energy source
Provide twice the amount of energy of carbohydrates or proteins, 37KJ per gram of food
Advantage is large amounts of energy need to be consumed in small masses of food
What is the structure of phospholipids
Made up of glycerol, a phosphate group and fatty acids
The fatty acid tails are bonded to the glycerol by ester bonds
Phosphate head is hydrophilic, polar and soluble in water
Fatty acid tails are hydrophobic, non-polar and insoluble in water
They are amphipathic - have both hydrophobic and hydrophilic pats
What is the function of cholesterol
Important for the structure of many cells membranes
Also the starting point for the synthesis of steroid hormones such as
Oestrogen
Progesterone
Testosterone
Bile salts: involved in lipid digestion and assimilations are also formed from cholesterol
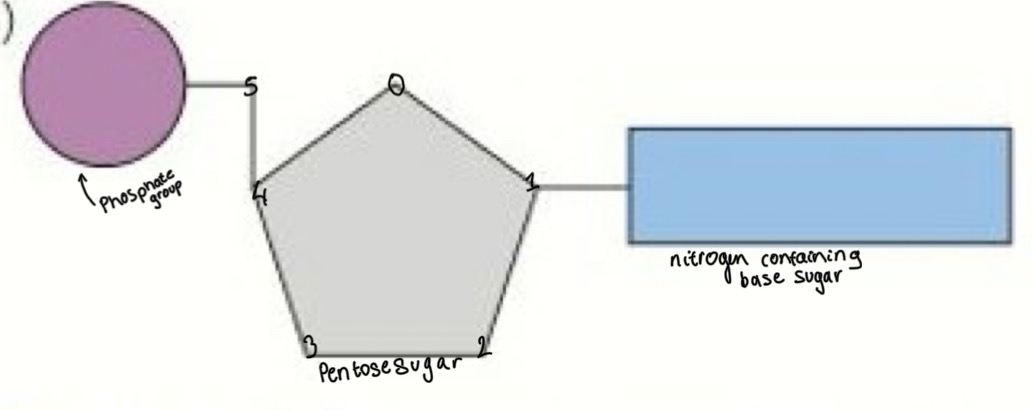
What are nucleotides
Nitrogen-containing organic substances that form the basis of (they are the monomers) the nucleic acids DNA + RNA
Draw, label and explain the nucleotide structure
2 condensation reactions:
Base and sugar form a glycosidic bond
Phosphate and sugar form an ester bond
How are the 5 nitrogenous bases organised
Purine bases (double ring structure):
Adenine (A)
Guanine (G)
Pyramidine bases (single ring structure):
Thymine (T)
Cytosine (C)
Uracil (U)
How is a polynucleotide formed
Nucleotides (N) are linked together by condensation reactions
The phosphate group at the 5th carbon of the pentose sugar at one N forms a covalent bond with the hydroxyl group (OH) at the 3rd carbon on the adjacent N
The bonds formed are called phosphodiester bonds
These form long chains called a sugar phosphate backbone
How do polynucleotides form the double helix in DNA
Two parallel strands are arranged so that they run in opposite directions - antiparallel
The bases hold the two strands together via hydrogen bonds
The strand on the left is the 5’3 strand and the strand on the right is the 3’5 strand
A & T are able to form 2 hydrogen bonds
C & G are able to form 3 hydrogen bonds
They can only bind to each other - complementary base pairing
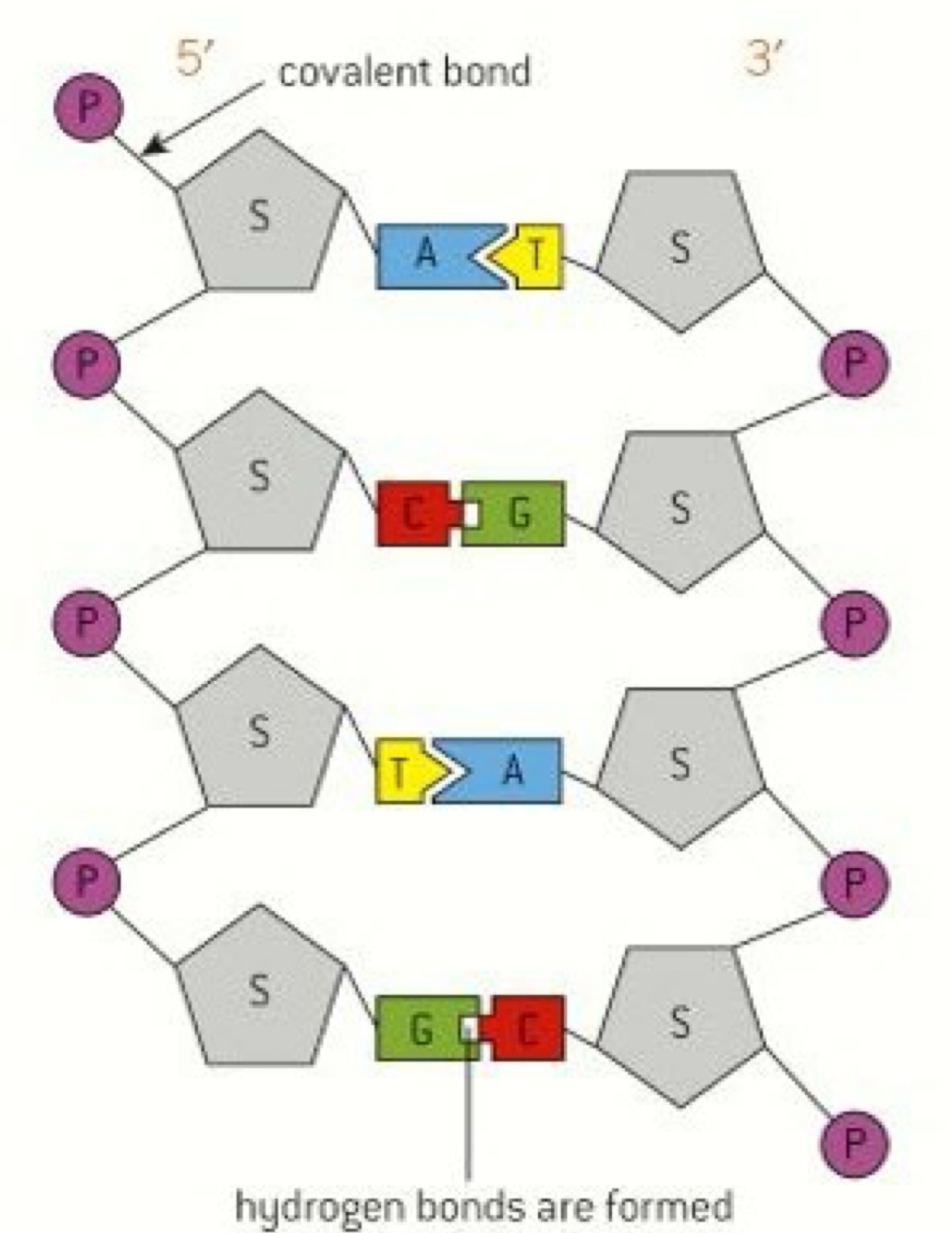
What are the base pairing rules
A small pyrimidine will always bond to a larger purine base
This arrangement causes a constant distance between the DNA ‘backbones’, resulting in parallel chains
Complementary base pairings mean that DNA always has equal amount of adenine and thymine and equal amounts of cytosine and guanine
Differences between DNA and RNA (4)
DNA is Deoxyribose pentose sugar while RNA is ribose pentose sugar
DNA uses thymine while RNA uses uracil
DNA is double stranded while RNA is single stranded
RNA is also used in mRNA and eRNA while DNA isn’t
Similarities between DNA and RNA (3)
Both contain phosphate group, pentose sugar and nitrogenous base
Both form via 2 condensation rections
Both contain phosphodiester bonds
How is DNA packaged into cells
DNA is large so when it is stored in a cell it is wound around histone proteins - giving chromosomes.
Therefore each DNA molecule gives one chromosome.
DNA without histones can also be found in
mitochondria and chloroplasts
Prokaryotes
DNA is naked: not in a nucleus and it
is not wound round histones
Some viruses also contain naked DNA
Explain semi conservative replication
DNA helicase uncoils and breaks the hydrogen bonds
Double helix unwinds
Free activated DNA nucleotides join the unpaired bases. DNA polymerase rejoins the sugar phosphate backbone
Hydrogen bonds form
Phosphodiester bonds form between nucleotides
Half the molecules is old DNA, half is made of new molecules - hence semi conservative
The leading strand (3-5) is replicated continuously while the lagging strand (5-3) is replicated in short fragments which are later joined by DNA ligase
A winding enzyme winds the new strands up to form
double helices
The two new molecules are identical to the old molecule