3.0 glucose and its metabolism
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
catabolism
the breakdown
anabolism
synthesis
metabolism =
catabolism + anabolism of biochemical compounds occurring via separate enzymatic routes
catabolism
the production of cellular energy in the form of ATP
reducing powers in the form of NADH, FADH2 or NADPH.
Anabolism consumes this reducing power and ATP
to build new molecules
Within the body, we observe a continual switching between catabolic and anabolic pathways in order to maintain homeostasis.
A key element in this metabolic switching is a reciprocal regulation of opposed pathways catalysing opposing processes.
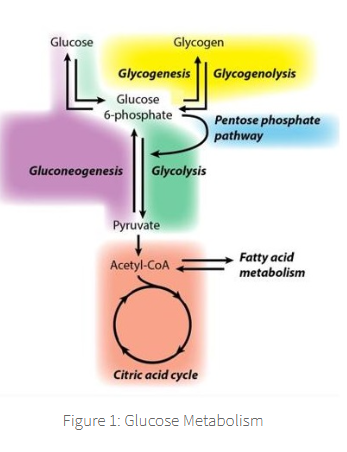
It is useful to think of glucose metabolism as linked reactions occurring in blocks,
where set enzymatic reactions occur
Each block generates molecules that are then required for the next block,
untimely generating energy (ATP) through aerobic catabolism in the Citric Acid Cycle.
The process is started through carbohydrate digestion in the GI tract → free circulating glucose,
free circulating glucose is up taken into cells for it's metabolism,
AKA glycolysis. (glucose → into cells as glycogen)
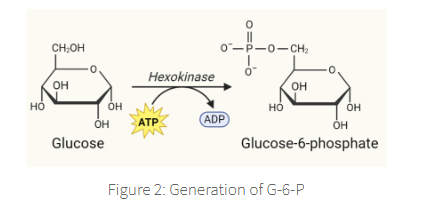
The primary step in glycolysis is the phosphorylation of glucose by hexokinase and glucokinase
results in the formation of glucose-6-phosphate (G-6-P),
which is able to inhibit hexokinase but not glucokinase
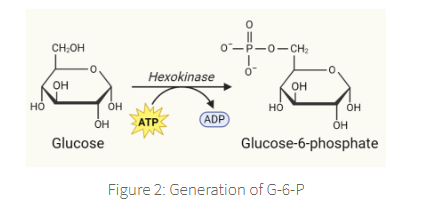
This phosphorylation step is essential
in order to prevent diffusion of glucose back outside the cell.
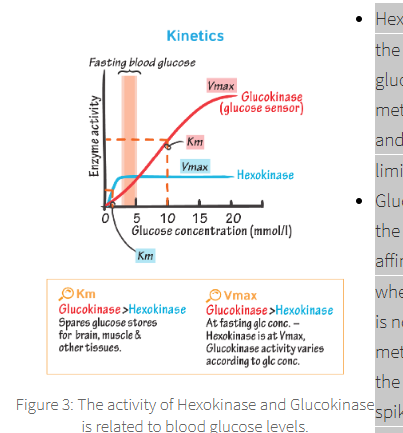
these two enzymes show activity at differing concentration of fasting blood glucose:
Hexokinase,
Glucokinase,
Hexokinase is broadly expressed throughout the body, including the liver,
shows a high affinity for glucose (low Km),
highlights it as the metabolic enzyme for maintenance of background + average blood glucose.
Km
the concentration of a substrate that results in a reaction rate of half its maximum velocity
hexokinase RoR is limited
due to its inhibition by G-6-P.
Glucokinase,
which is only expressed in the liver and the β-cell of the pancreas,
glucokinase shows a much lower affinity for glucose (high Km),
indicating activity only when high levels of blood glucose are present.
As it is not rate limited by G-6-P Glucokinase will remain metabolising glucose
even as G-6-P accumulates in the liver cells
G6P is therefore crucial during glucose spikes.
In pancreatic cells glucokinase ultimately results in the release of insulin.
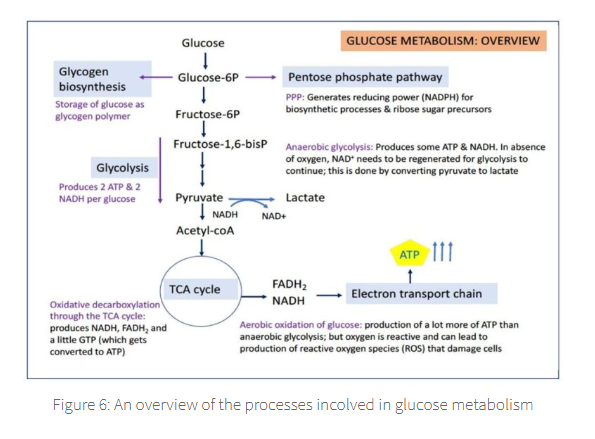
G-6-P then is either:
Biosynthesised → Glycogen
Metabolised via the Pentose Phosphate pathway to generated → NADPH
Metabolised to generate → pyruvate via glycolysis to informs the citric acid cycle, ultimately generating →lactate