Unit 1 summary
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
71 Terms
What does DNA code for?
the sequence of amino acids in the primary structure of a protein
Diagram of the structure of a nucleotide
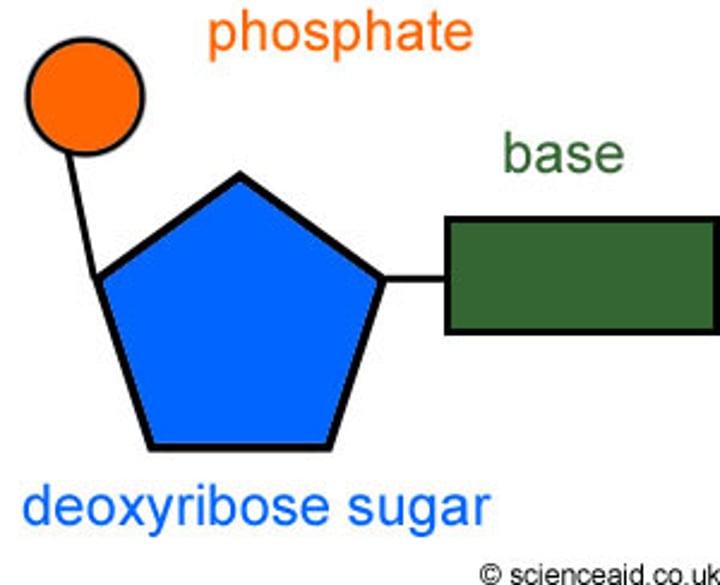
How are polynucleotides formed?
via condensation reactions b/w the deoxyribose sugar and the phosphate group
- creates a phosphodiester bond
(sugar-phosphate backbone)
How is the double helix formed?
- The hydrogen bonding between the polynucleotides (A-T/C-G)
- the coiling of the polynucleotides
What is complementary base pairing?
When base A always pairs up with base T, and base C always pairs up with base G
Which bases are purines?
Adenine (A) and Guanine (G)
they are 2-ring purine bases
Which bases are pyrimidines?
cytosine, thymine, uracil
they are 1 ring pyrimidine bases
How many H bonds are between A and T?
2 H bonds
How many H bonds are between C and G?
3 H bonds
what does complementary base pairing do?
complementary base pairing helps maintain the order of the genetic code once DNA is replicated
Explain why DNA replication is semi-conservative and its importance within the body.
Produces 2 genetically identical DNA copies
Each new DNA molecule contains 1 new strand and 1 original strand
Ensures that new cells have identical genetic material to original cells
name 6 functions of DNA due to its structure
1.
stable structure due to the sugar-phosphate backbone and double helix
2. two stranded, so both strands can act as templates
3. weak hydrogen bonds between bases allow two strands to separate / unzip during DNA replication
4. double-stranded for semi-conservative replication
5. large molecule to carry lots of info
6. stable structure due to the sugar-phosphate backbone and double helix
stable structure due to the sugar-phosphate backbone and double helix
the role of the 2 enzymes in semi-conservative replication of DNA
DNA helicase causes the breaking of H-bonds between DNA strands
DNA polymerase joins the DNA nucleotides, forming phosphodiester bonds
contrast the structures of ATP and a nucleotide found in DNA
ATP has ribose whereas DNA nucleotide has deoxyribose
ATP has 3 phosphate groups whereas DNA nucleotide has 1 phosphate groups
ATP base is always adenine whereas the base in DNA nucleotide can vary (e.g. C,G and T)
explain how the active site of an enzyme causes a high rate of reaction
lowers activation energy by weakening/bending bonds
induced fit causes active site of an enzyme to change shape
ESC causes bonds to weaken
Explain the induced fit model
Before reaction: enzymes active site not complementary to shape of substrate
1. Substrate binds to active site/enzyme
2. E-S complex forms
3. Active site changes shape, distorting/breaking bonds in the substrate
4. Active site changes shape so its complementary to substrate
Explain the effect of increasing the concentration of substrate on the rate of reaction without inhibitor
increasing the concentration of substrate increases the rate of reaction up until a certain point:
rate of reaction will increase with increases substrate
increasing substrate means that more molecules available to enter active site
more substrate molecules enter active site
more substrate binds to the enzyme active site
more successful collisions
forming more ESC
however at a high concentration of substrates, it reaches a point where all the active sites are occupied
no further increase in rate (of reaction)
at high substrate concentration, the enzyme concentration is limiting
Explain the effect of increasing the concentration of substrate on the rate of reaction with inhibitor
the inhibitor has a similar shape to substrate
the inhibitor binds to the enzyme active site instead of the substrate, occupying it temporarily
therefore fewer active sites for substrates
explain why different enzymes are involved in each stage of the digestion process
enzymes are specific
the substrates are different shapes
active site and substrate are complementary
so ESC forms
explain why the activity of an enzyme falls to zero at a pH of 7
the pH is too high/acidic
change in pH alters charge distribution on enzyme molecule
change in charge of active site and changes shape of active site
H-bonds disrupted, which changes tertiary structure
changes shape of active site
substrate no longer fits active site
substrate cannot bind to active site
ESC cannot form
RoR decreases
explain why the initial rate of reaction with enzymes is greater than you may calculate
conc. of substrate is higher at the start
so more chance of substrate entering active site
so most active sites become occupied
explain enzyme action at an optimum temperature
molecules have kinetic energy
frequent collisions between enzyme and substrate molecules
more ESC formed
max rate (of reaction) achieved
explain enzyme action at a temperature higher than the optimum temperature
molecules have an increase in kinetic energy
collisions occur more frequently and with more energy
molecules vibrate
which breaks bonds
therefore hydrogen bonds broken
tertiary structure/3D shape of enzyme changed/altered
active site changes shape and loses its complementary shape
enzymes are denatured
substrate molecules no longer fits into active site
will decrease rate (of reaction)
may be irreversible
if this is the case then the reaction stops
suggest how plasmodium obtains amino acids from haemoglobin with red blood cells
hydrolysis of haemoglobin by enzymes
which breaks peptide bonds
Sucralose is a chemical that has been made from sucrose by replacing three of the hydroxyl (OH) groups with Chlorine (Cl) groups.
Suggest why sucralose cannot be digested in the body
the Cl on the sucralose instead of OH gives it a different shape/structure
enzymes are specific and only act on one substrate
no suitable enzyme present in the body to digest sucralose
so therefore no ESC formed
effect on enzyme concentration on rate of reaction
reaction rate increases with increased enzyme
more reaction sites available
the rate (of reaction) will decrease as substrate is used up/becomes limiting
effect of competitive inhibitor on rate of reaction
competitive inhibitor has similar shape to substrate
so it can fit/occupy active site
for a short period of time/temporarily/reversibly
competitive inhibitor complementary to active site
competitive inhibitor binds/attaches to enzyme active site instead of substrate
prevents/blocks substrate from entering active site
effects can be reversed by increasing substrate concentration
effect on non-competitive inhibitor on rate of reaction
reduces rate (of reaction)
fits into allosteric site/site other than active site
alters/changes shape of active site
substrate cannot fit/bind to allosteric site
increasing substrate concentration has no effect
general structure of amino acid
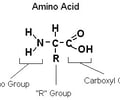
describe how 2 amino acids are joined together to form a dipeptide.
- formed via condensation reaction
- water is removed
- peptide bond forms b/w the carboxylic group of one amino acid and the amine group of the other amino acid
Describe how the structure of a protein depends on the amino acids it contains.
The structure of a protein is determined by the position of amino acids
the primary structure is sequence of amino acids (in the polypeptide chain)
the secondary structure is the folding of the polypeptide chain
H-bonds form between NH group of one amino acid and C=O group, to form alpha helix and beta-pleated sheets
the tertiary structure is formed by interactions (ionic bonds, hydrogen bonds, disulfide bridges) between R groups
creates the active site in enzymes
the quaternary structure contains more than 1 polypeptide chain
formed by bonds b/w polypeptides
may involve the addition of a prosthetic group
Each amino acid has a different R group.
Describe how these R groups can interact to determine the tertiary structure of a protein.
Some R groups attract
Disulfide bridges between S atoms
Ionic bonds between oppositely charged R groups
Hydrophyllic R groups on the outside of molecules
Hydropgobic R groups on the inside of molecule
Two proteins have the same number and type of amino acids, but have different tertiary structures.
Explain how.
different sequence of AA
forms ionic/hydrogen bonds in different places
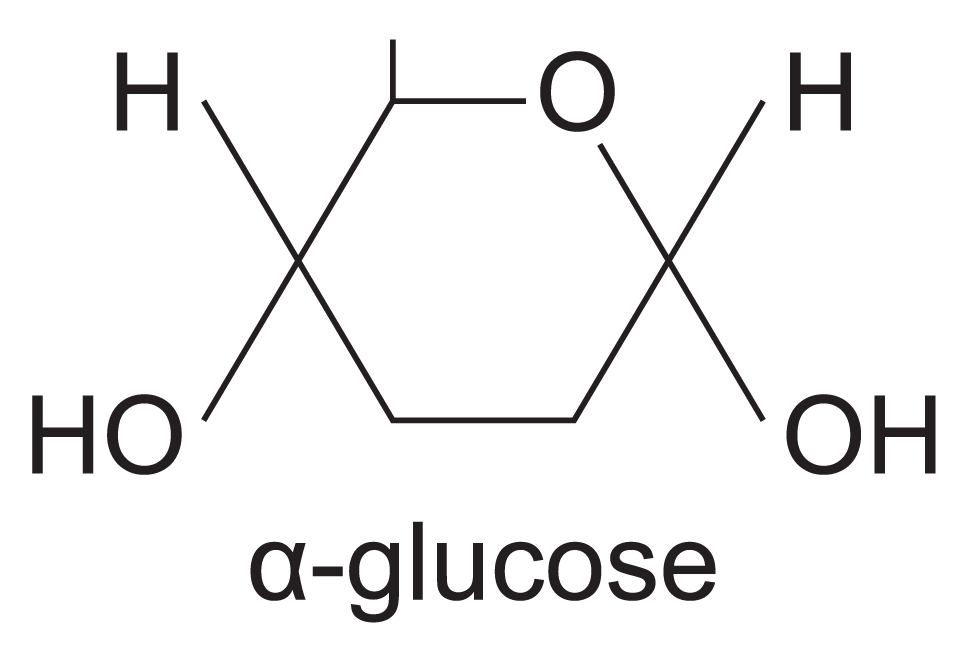
structure of alpha glucose
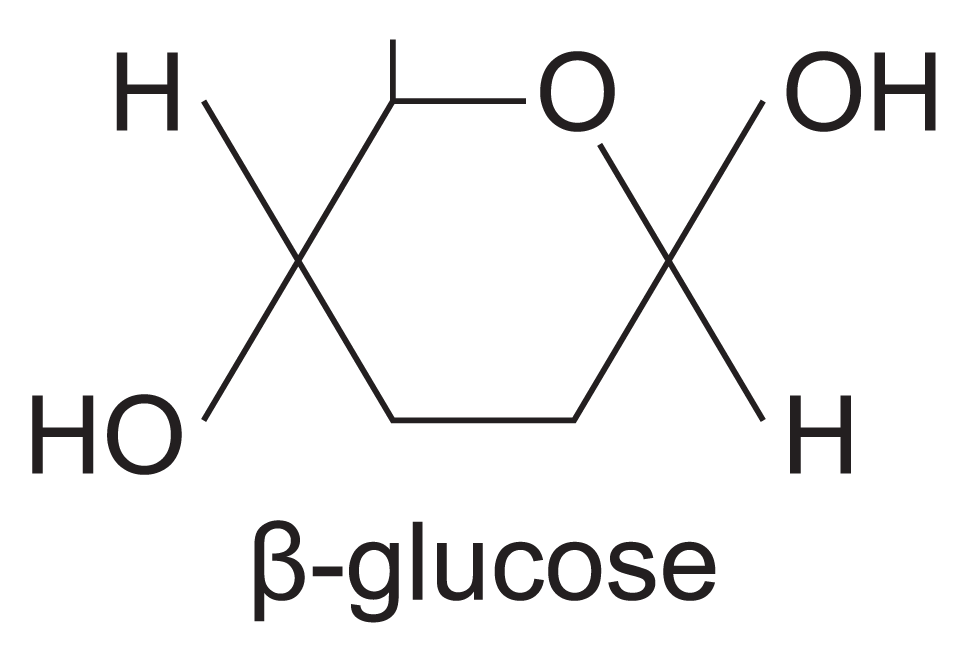
structure of beta glucose
difference between alpha and beta glucose
Position of hydrogen and hydroxyl groups on carbon atom 1 inverted.
word equation of maltose
Glucose + Glucose -> Maltose + Water
word equation for lactose
glucose + galactose -> lactose + water
word equation for sucrose
glucose + fructose --> sucrose + water
ways in which starch molecules are adapted for their functions in plant cells
insoluble
doesnt affect water potential;
Helical
Compact
Large molecule
Cannot leave cell.
features of starch that make it a good storage molecule
Insoluble (in water)
so doesn’t affect water potential;
Branched / coiled / (α-)helix
so makes molecule compact/can fit many molecules in a small area
Polymer of α glucose
so provides glucose for respiration
Branched / more ends
for fast breakdown / enzyme action
describe the structure of a cellulose molecule and explain how cellulose molecules are adapted for their function in plant cells
made from beta glucose
joined by condensation reaction
forms 1-4 glycosidic bonds
Long and straight chains
Become linked together by many hydrogen bonds to form
fibrils
Provide strength to cell walls
cellulose makes cell walls strong and can resist turgor pressure
structure of glycogen
Polysaccharide/polymer of α-glucose
joined by glycosidic bonds
branched structure
suggest how glycogen acts as a source of energy
hydrolysed to glucose
glucose is used in respiration
describe the differences between the structure of a cellulose molecule and a glycogen molecule
Cellulose is made up of β-glucose (monomers) and glycogen is made up of α-glucose (monomers)
Cellulose molecule has straight chain and glycogen is branched
Cellulose molecule has straight chain and glycogen is coiled
4. glycogen has 1,4- and 1,6- glycosidic bonds and cellulose has only 1,4- glycosidic bonds;
describe how the concentration of a reducing sugar can be measured using a calorimeter
using a known concentration of reducing sugar:
heat w benedcits solution
use same volume of solution each time
use an excess of Benedicts
solution changes colour to brick red
remove precipitate/obtain filtrate
calibrate using water/unreacted benedicts
use red filter
read the transmission/absorbance
more transmission/less absorbance = more sugar present
obtain calibration curve by plotting transmission/absorbance against reducing sugar concentration
use the reading of the unknown sugar solution and read off the graph to find the concentration
how should a procedure to test for reducing sugars be adapted to test for non-reducing sugars
hydrolyse non-reducing sugar w acid
Non-reducing sugar test
So Benedict’s test: colour stays blue
Boil with acid then add acid to neutralise
Heat with Benedict’s solution
Colour change from blue to red
what does the term electronegativity mean with regards to a water molecule?
oxygen is electronegative and pulls the shared electrons towards it
- the opposite side of the hydrogen becomes 𝛅+
- the opposite side of the oxygen becomes 𝛅-
diagram of water molecules bonded together
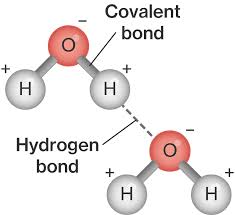
properties of water that help organisms survive in a pond
water has a high SHC
so water buffers changes to temperature
lots of energy required to break hydrogen bonds
so temperature doesn’t change much
can take a lot of heat without changing temperature
polar molecule
acts a universal solvent
Water is a solvent
so metabolic reactions can occur
water can act as a solvent for ions such as nitrates
water is reactive
takes places in hydrolysis
water is a metabolite in condensation/hydrolysis/photosynthesis/respiration
large latent heat of vaporisation
it takes a lot of energy to break the hydrogen bonds between the water molecules
a lot of energy is used up when water vaporises
so provides a cooling effect through evaporation
cohesion between water molecules
so supports columns of water in plants
so produces surface tension, supporting small organisms
gives support to large bodies
Ice floats
ice forms an insulating layer
so organisms don’t freeze and so organisms can still swim
water expands as it freezes
cooling allows max no. of hydrogen bonds to form
water molecules space out to allow this
water is transparent to light
so photosynthesis possible (in shallow waters)
explain how there's a strong cohesion b/w water molecules
water molecules are cohesive because they are polar
how does water help in control of body temperature?
heat energy is removed when water evaporates from the skin
- this lowers the internal temperature
monomer definition
a repeating unit from which larger molecules are made
polymer definition
lots of monomers bonded together
The chemical reactions involved in the conversion of monomers to polymers and polymers to monomers, including examples
a condensation reaction joins monomers together to form polymers and forms a chemical bond and releases water
e.g. proteins are made from amino acids and are joined together via condensation reactions, forming a peptide bond
a hydrolysis reaction breaks the bond between monomers and uses water
e.g. starch is made from glucose which are joined by condensation reaction, forming glycosidic bonds
What is the role of ATP?
an immediate energy source for biological processes
structure of ATP
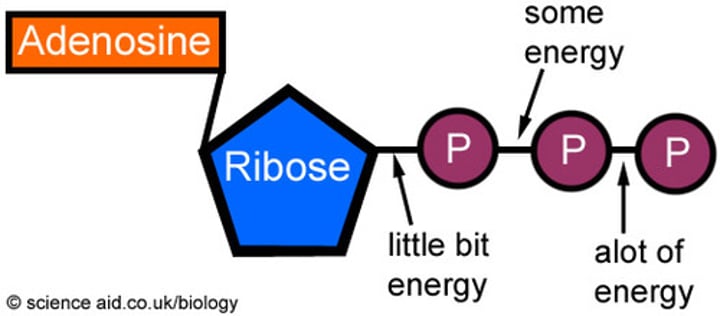
How is ATP made?
Adenine, ribose and 3 phosphates join together by condensation reaction
- catalysed by ATP synthase
How is ATP broken down?
via hydrolysis:
- ATP can be hydrolysed to ADP and Pi (inorganic phosphate)
- catalysed by ATP hydrolase
what happens when ATP is hydrolysed?
a phosphate bond is broken and energy is released
Why is ATP an immediate energy source?
because only 1 bond has to be hydrolysed for energy to be released
give 4 reasons why ATP is suitable as the 'energy currency' of cell.
1. ATP has high energy bonds b/w the phosphate groups
2. only small amounts of energy are released at a time
- so less energy is wasted as heat
3. energy is available quickly due to single-step hydrolysis
4. ATP is readily resynthesised
explain how ATP can transfer energy to different compounds
the inorganic phosphate released during the hydrolysis of ATP can be bonded to different compounds to make them more reactive
- this is known as phosphorylation
explain 5 properties of ATP as an energy source, in comparison to glucose.
1. ATP releases energy in small, manageable amounts so that no energy is wasted
- prevents cells from overheating due to wasted thermal energy
Glucose releases large amounts of energy
- results in lots of wasted energy
2. ATP is a small and soluble molecule that can move around cytoplasm w ease
so it's easily transported around the cell
THIS IS THE SAME FOR GLUCOSE
3. ATP is an immediate energy resource b/c only 1 bond has to be hydrolysed for energy to be released
IN COMPARISON:
several glucose bonds have to be broken down for glucose to release energy
4. ATP can transfer energy to another compound by transferring one of it's phosphate groups
- this is bc ATP can enable phosphorylation
- this makes the compound more reactive
IN COMPARISON:
- glucose cannot do this bc it does not contain phosphate groups
5. ATP can't pass out of the cell, so the cell always has an immediate supply of energy
IN COMPARISON:
glucose can leave the cell via channel and carries proteins
- so a cell can run out of glucose but it can't run out of ATP
Describe how you would test a piece of food for the presence of lipid
crush and dissolve in alcohol, then add water
white emulsion would show presence of lipid
explain why triglycerides are not considered to be polymers
not made of monomers
Humans synthesise more than their body mass of ATP each day. Explain why it is necessary for them to synthesise such a large amount of ATP.
ATP cannot be stored
ATP only releases a small amount of energy at a time
describe how ATP is made in mitochondria
ATP produced in krebs cycle
krebs cycle produces NADH
electrons released from NADH
electrons pass through ETC, which releases energy
ADP + Pi ——→ ATP
by ATP synthase
Describe the part played by the inner membrane of a mitochondrion in producing ATP
electrons transferred down ETC
this provides energy to take protons into the space between the membranes
protons pass back, through the membrane, into the matrix
energy used to combine ADP + Pi
compare and contrast the structure and properties of triglycerides and phospholipids
both contain ester bonds
both contain glycerol
the fatty acids on both may be either saturated or unsaturated
both are insoluble in water
both contain C,H and O
however, phospholipids also contain P
triglyceride has 3 fatty acids and phospholipids have 2 fatty acids and phosphate group