Progeria and Clinical trials (Dr. Smallwood)
1/35
Earn XP
Description and Tags
NO PHARAMCOKINETICS
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What is Progeria
Progeria, also known as Hutchinson-Gilford Progeria Syndrome, is a rare genetic disorder characterized by accelerated aging in children, resulting in symptoms such as growth failure, hair loss, and cardiovascular problems.
What type of inheritance is progeria
The mutation is autosomal dominant
Progeria patients have one normal allele (one normal copy of the LMNA gene) and one mutant allele
One copy of the mutant allele is enough to cause progeria
What differentiates classical progeria from progeria
Classical Progeria is caused by a mutation of the LMNA gene
Mutated LMNA gene - Abnormal lamin A protein (progerin) - Progeria
LMNA gene should just produce Lamin A protein
What gene is affected in progeria
The LMNA gene is affected in progeria, which encodes the protein lamin A.
Mutations in this gene lead to the production of an abnormal version of the protein, resulting in accelerated aging symptoms.
What changes happen to the progeria nuclei compared to normal
Filament protein
Forms basketwork structure called the nuclear lamina
Located just inside the inner nuclear membrane
Provides structure and shape to the nucleus
In progeria lamin A protein (progerin) is damaged, does not form the correct basketwork structure and gives progeria nuclei an abnormal shape
What are the normal Lamin A interactions
Lamin A interacts with many other proteins and chromatin
Essential for normal gene transcription
However: these interactions can go wrong in progeria
What are some phenotypes realted to progeria
Heart disease
Low weight
Short stature
Low body fat
Aged skin
Osteoporosis
Osteolysis
Joint stiffness
Hair loss
Where does the muations for Lamin A on LMNA gene happen
Classical progeria mutation is located in exon 11 in the LMNA gene
LMNA gene mutation: c.1824C>T
Lamin A protein mutation: p.G608G (GGC>GGT), mostly just written G608G
What does alternative splicing mean in progeria
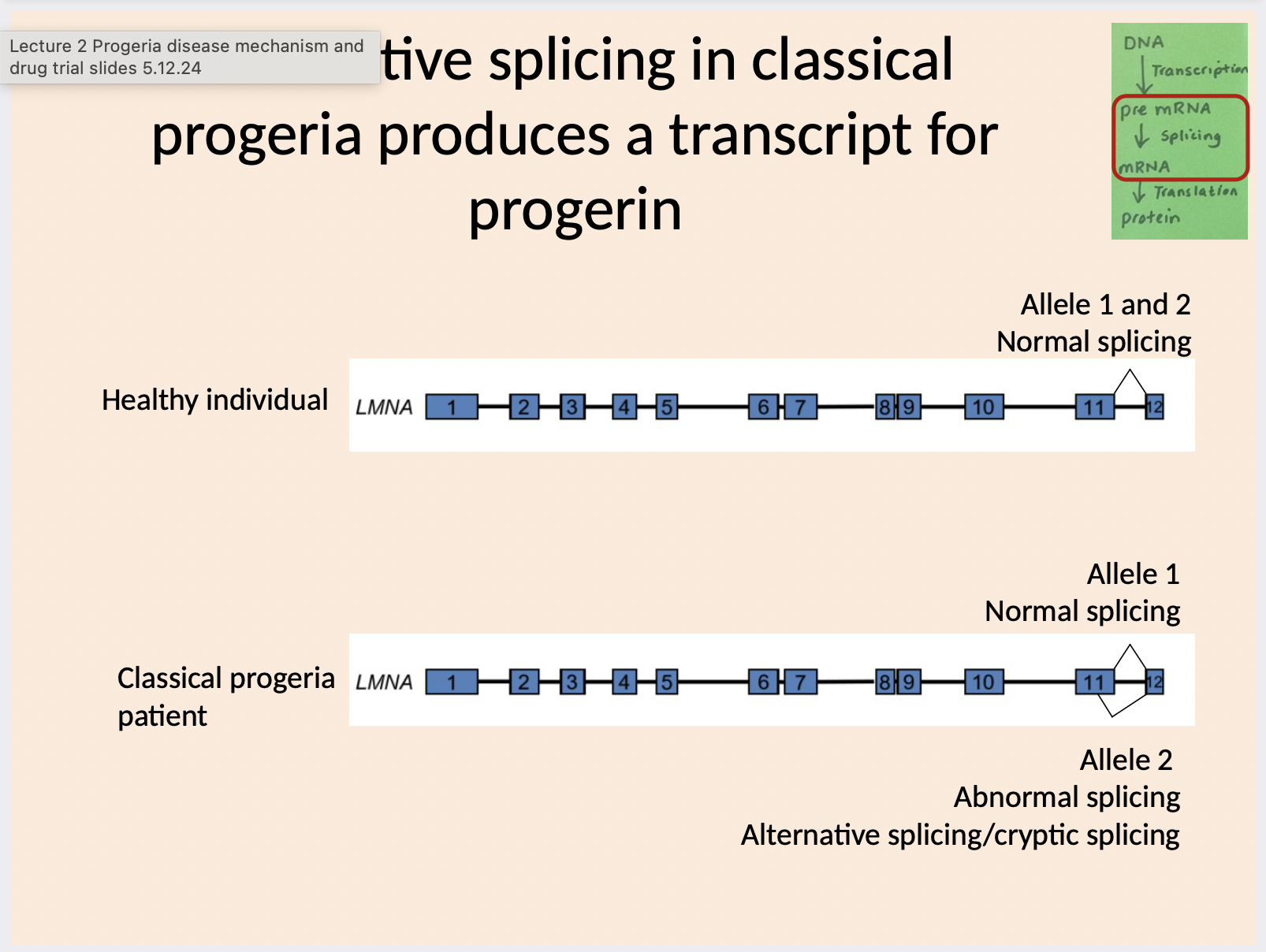
Which exons are involved in alternative splicing in progerin
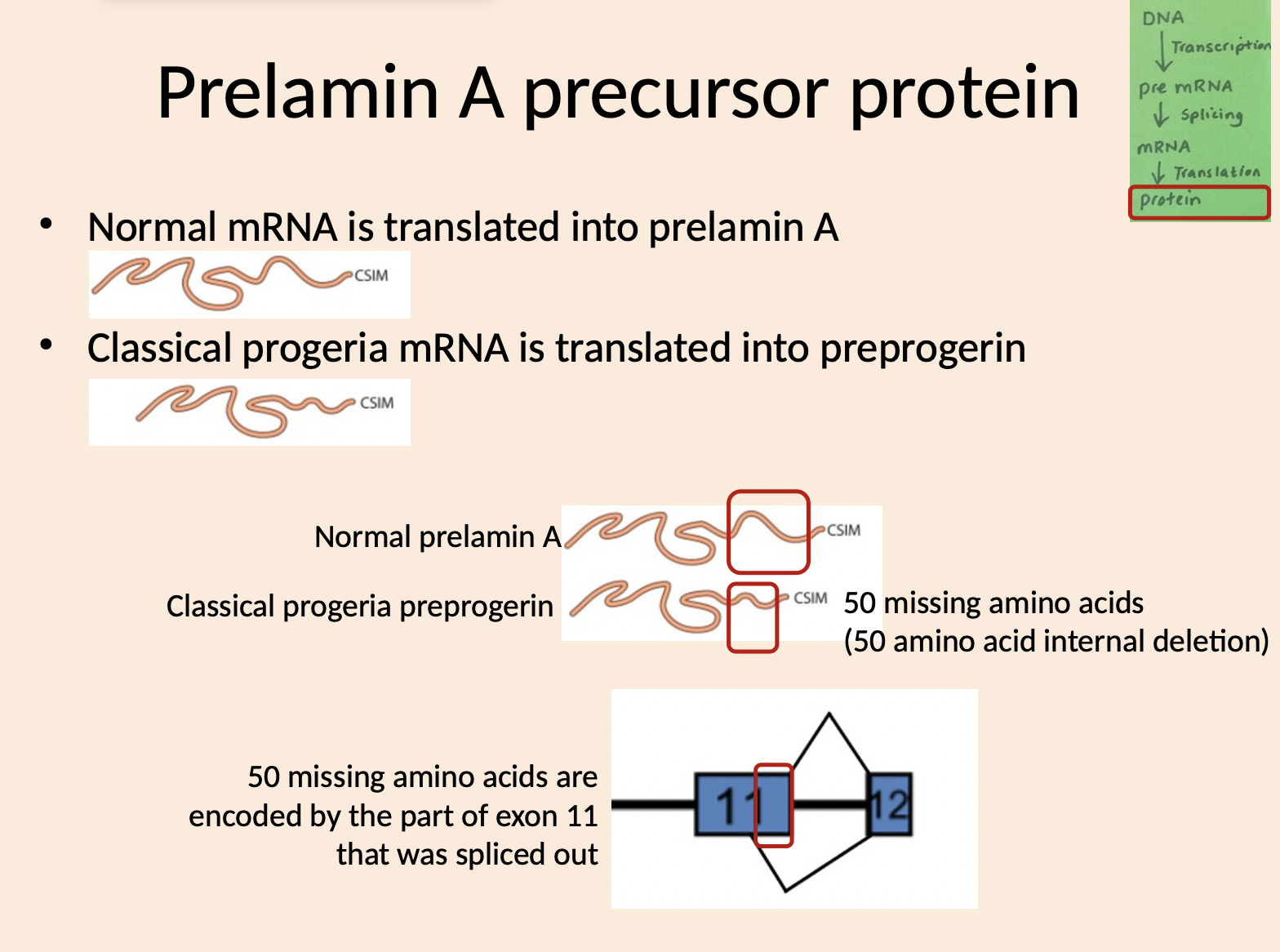
How many amino acids are deleted
50 missing amino acids
(50 amino acid internal deletion
What events occur in post-translational processing
Common method of targeting proteins to membranes is to add a lipid group
Farnesyl group is one type of lipid (there are many)
What is the sequence of normal events for prelamin A to mature lamina A
Prelamin A C-terminus ends in 4
amino acids (CSIM)Enzyme farnesyltransferase adds a
farnesyl (lipid) group to amino acid CNext there are 2 cleavage steps by
enzyme ZMPSTE24Cleavage 1 removes the 3 C-
terminal amino acids SIMCleavage 2 removes a further C-
terminal 15 amino acids including the farnesyl groupProcessed protein is mature lamin A
What is the sequence of abnormal events for prelamin A to mature lamin A
Preprogerin C-terminus ends in 4 amino acids (CSIM)
Enzyme farnesyltransferase adds a farnesyl (lipid) group to amino acid C
Next there is 1 cleavage step by enzyme ZMPSTE24
Cleavage 1 removes the 3 C-terminal amino acids SIM
Cleavage 2 cannot happen because 2nd ZMPSTE24 binding site is absent due to 50 amino acid internal deletion
The farnesyl group remains attached
Processed protein is progerin (LAD50) / Preprogerin (614 amino acids)
What is lonafarnib drug
An inhibitor of farnesyltransferase used in clinical trials to treat progeria, aiming to prevent the farnesylation of prelamin A.
What are clinical trials
Research studies that evaluate the safety and effectiveness of new treatments or drugs in humans before they receive regulatory approval.
What are randomised controlled trials (RCTs)
A type of clinical trial that randomly assigns participants into experimental or control groups to compare outcomes and assess the efficacy of treatment.
What is the purpose of randomized controlled trials (RCTs)
The purpose of randomized controlled trials (RCTs) is to determine the effectiveness of a treatment by eliminating biases, ensuring random assignment to groups, and comparing outcomes between the experimental and control groups.
What is the Health research authority (HRA)
An organization that oversees ethical standards and regulations for research involving human participants in the UK, ensuring proper conduct and protection of rights.
What is the Research ethics committee
Participant information sheets provide clear information on involvement/risk/withdrawal and complaints
Informed consent occurs
Participants understand how their data and samples will be used, stored and shared
Ethical approval and regulation – informed
consent of participants
What is the phase I trial
First trial in humans
Small number of healthy volunteers
Is the drug toxic?
What dose is safe?
Are there side effects and are they acceptable?
Small numbers of patients
Minimum therapeutic dose (lowest effective dose)
What are side effects and why are they there
Side effects are unintended consequences of a drug treatment, resulting from the drug's interaction with biological processes.
They may arise due to the mechanism of action, dosage, or individual patient factors.
What is the phase II trials
Several hundred patients
• Does the drug work?
• What dose is effective?
What is the phase III trials
Large numbers of patients (usually thousands)
How well does the new drug work?
Often (not always) RCTs:
new drug versus standard treatment
new drug versus placebo
What makes RCT gold standard
Controlled
Participants allocated into control or treatment groups using randomisation methods
Employ methods of bias reduction
Large numbers increase data quality
What is bias and its impact in clinical trials
Bias is prejudice for or against something
Bias makes clinical trial findings less valid:
Systematic error in trial design or conduct
Skews/deviates results from the truth
Can lead to under or over estimation of the effect of a treatment
What are the 4 types of bias
Allocation Bias
Performance Bias
Assessment Bias
Attrition Bias
Explain what is allocation bias
Bias in the way trial participants are allocated into groups
Trial staff are unlikely to be biased deliberately, bias can be unconscious
Believing a new treatment is better so unconsciously placing sicker patients in the treatment group
Knowing a drug has side effects so unconsciously avoiding putting sicker patients in the treatment group
How can we mitigate allocation bias
Simple randomisation
Assign participants randomly to control or treatment group
Reduces bias
50:50 chance of being in treatment or control group
Problems:
Smaller trials could end up with unequal numbers
Participant characteristics could influence results
Block randomisation
Method of allocating patients into treatment or control groups (usually by computer)
Prevents trial staff accidentally predicting the patient trial group by sequence
Covariates (participant characteristics not linked to response to the trial drug)
– Age
– Gender
– RaceConfounding factors (participant characteristics likely to influence response to the trial drug)
– Disease severity
– Co-morbidities
– Other specific drug treatments
What is performance bias
Participant response to treatment (performance) can be affected by:
Knowing their trial group
Trial groups receiving different care plans
Trial groups responding to expectations of trial staff
How do we minimise performance bias
Blinding - hiding the treatment group
Single blind - hiding group from participants
Double blind - hiding group from participants and trial staff
Treating all groups the same
Same care
Injections/tablets/tests appear identical for drug and placebo
What is assessment bias
Assessment of participant response to treatment can be affected by:
Trial staff or doctor assessment of participants is influenced by knowing the treatment group
Samples from different groups are assessed differently
How to Minimise:
Blinding
Standardise assessment methods and equipment
Use calibrated equipment
What is attrition bias
Participants drop out of a study
Can make group numbers unequal
Can reduce trial impact due to low participant number
What are ways to minimise attrition bias
Participation incentives
Good communication
Good trial management and organisation
What are phase IV trials
Drug has been approved and is for sale
Are there long term side effects?
Is the drug effective long term?
New drug
What other bias are there in clinical trials
Selection bias, performance bias, detection bias, reporting bias.