HYMS B9, W1
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
roles of the skin
- protection - UV, chemical, mechanical, thermal, pathogens, water
- sensation
- thermoregulation (sweat, vasodilation/constriction, hair, fat layer
- metabolic - vit d production
- sexual attractant (think beauty industry)
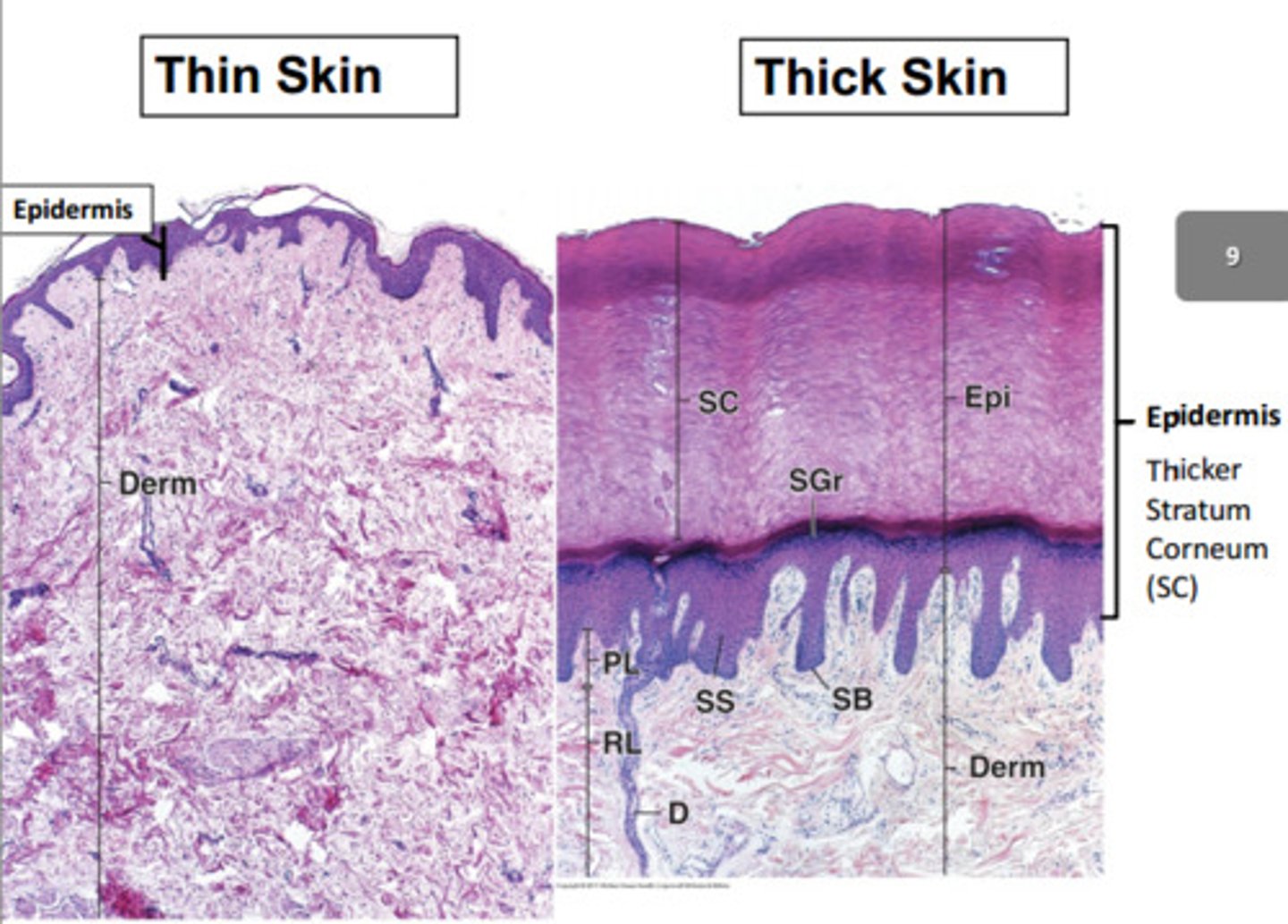
thick vs thin skin
- thin Skin (4 layers)
Less defined stratum corneum.
Less prominent rete ridges + dermal papillae.
Has hair follicles, sebaceous glands, arrector pili muscles.
Found most abundantly.
- thick skin (5 layers)
Stratum Lucidum present (an extra layers between the stratum granulosum and corneum).
More defined stratum corneum.
More prominent rete ridges + dermal papillae.
Hair and gland free.
Found on sole of feet + palm of hands.
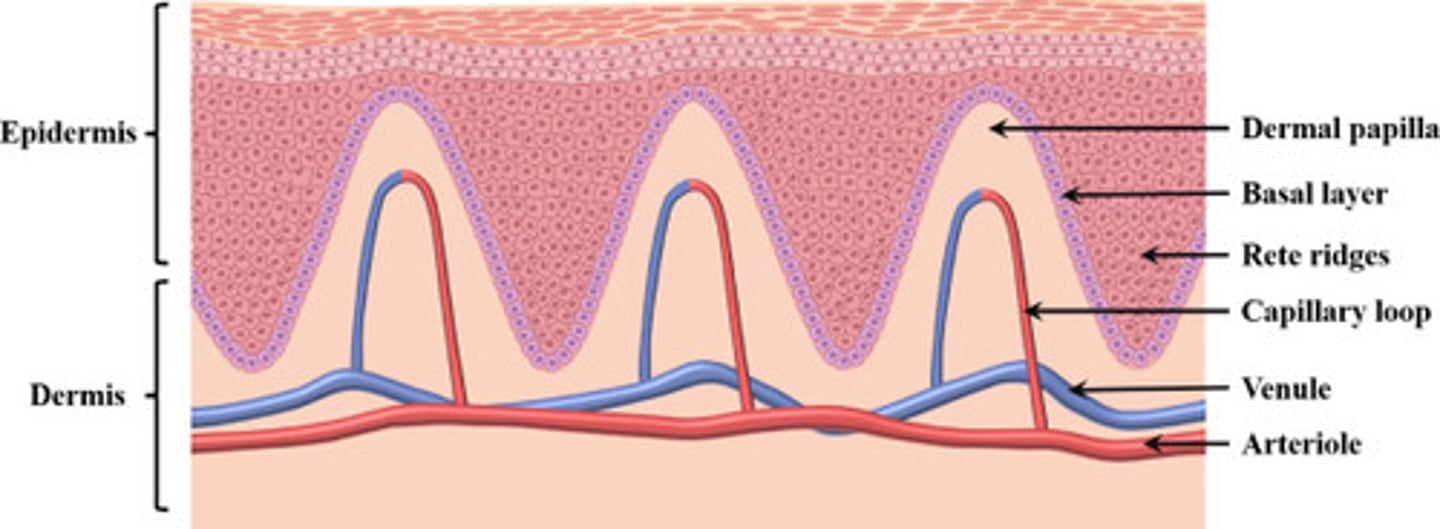
name two roles of the rete ridges and dermal papillae in the skin.
both increase the SA so that the dermis and epidermis attach to each other. this is especially important in thick skin which is why they are more prominent here.
both also increase the blood flow from the dermis to the epidermis (which has no supply of its own). this is important for the living cells of the epidermis.
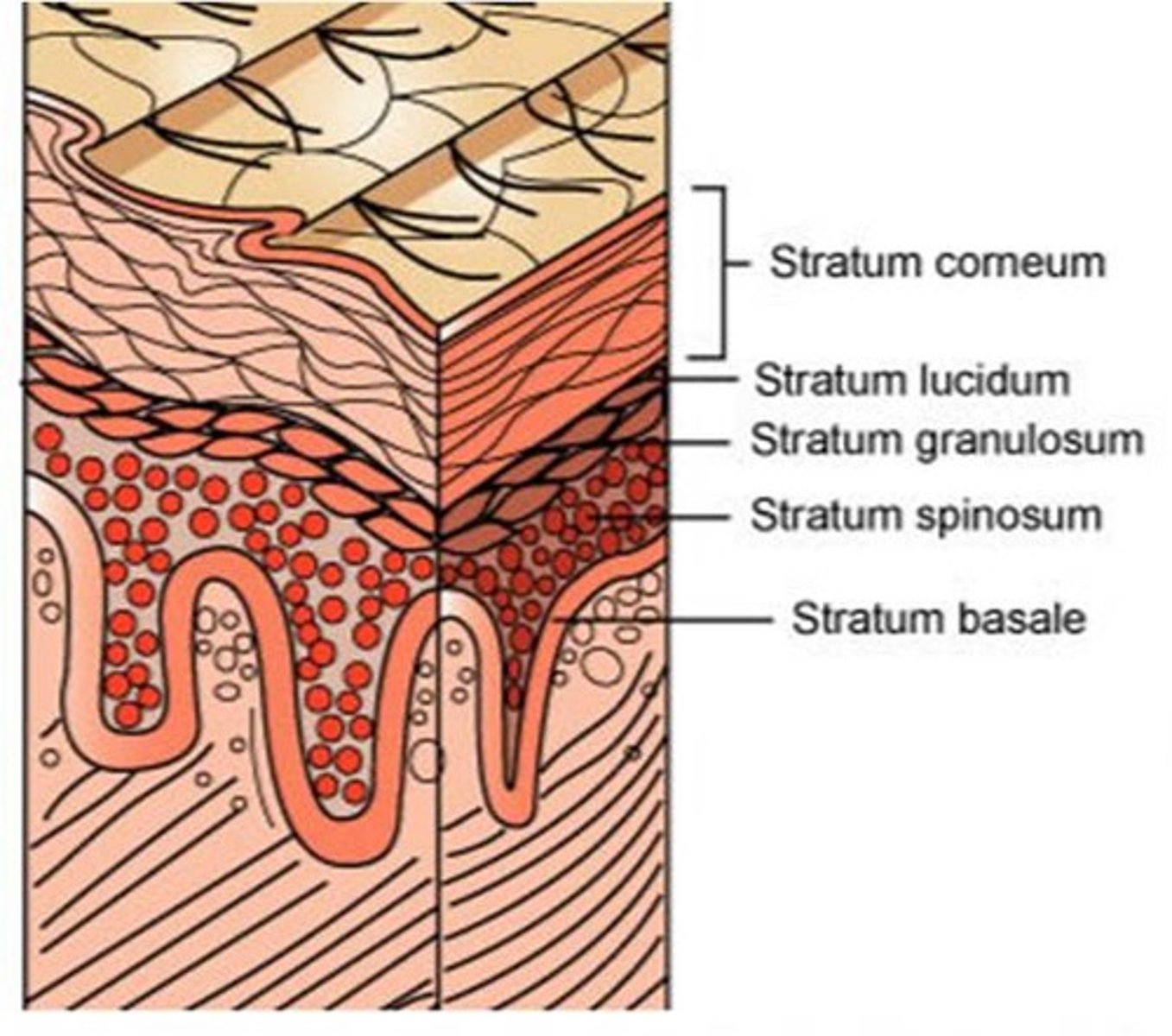
name the histological layers of the epidermis.
epidermis
1. stratum corneum
1.5. (in thick skin the stratum lucidum is present here).
2. stratum granulosum
3. stratum spinosum
4. stratum basale
dermis
describe the role of the stratum basale
- single layer of cells
- cuboidal or low columnar
- stem cells of the epidermis, divide by mitosis to produce the cells of the basal layer and the rest of the epidermal layer.
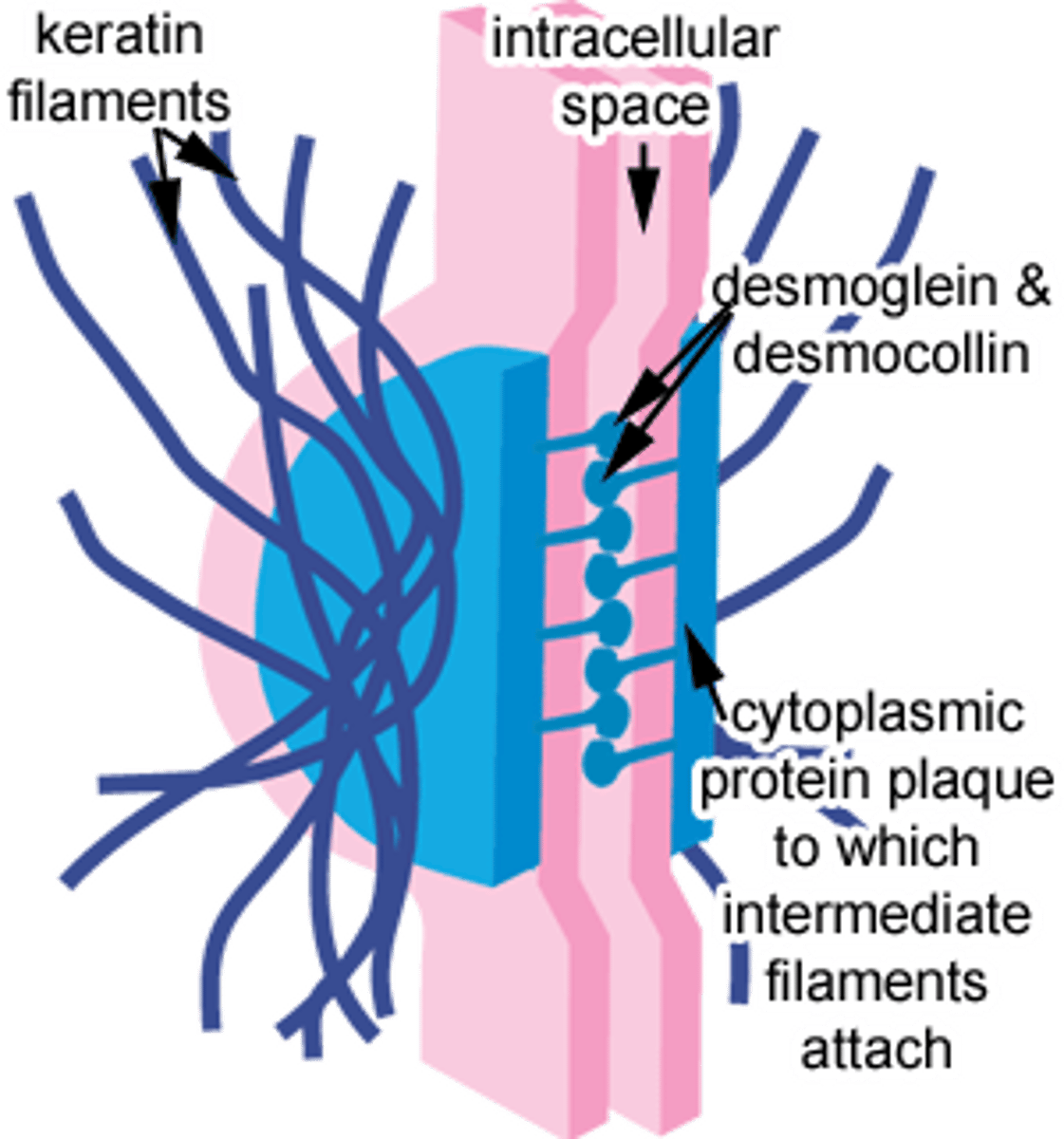
describe the histology of the stratum spinosum (aka prickle layer) in the epidermal layer of skin.
- several cells thick, becoming more flat.
- keratin filaments (type of intermediate filament of the cytoskeleton) bundle to form tonofibrils. tonofibrils converge and attach to desmosomes which are the cell junctions of the keratinocytes.
- cells here are no longer capable of cell division.
describe the histology of the stratum granulosum of the epidermis.
- cells become more flattened (squamous)
- they contain keratohyaline granules . kertohyaline + tonofibrils = keratin
- layer also contains lamellar bodies which contains lipids. important for hydrophobic barrier.
- at the surface of the granular layer, the cells lose their nuclei and cytoplasm leaving masses of keratin which coat the surface of the skin (aka stratum corneum).
what is the stratum corneum composed of?
flat flakes and sheets of keratin from apoptoses granular cells.
name three non epithelial cells in the epidermis and their role.
- melanocytes - protects against UV and gives skin colour. they synthesis melanin which is packaged into melanosomes. these vesicles are transferred into keratinocytes.
- Langerhans - APCs (present in all layers of epidermis but mostly in stratum spinosum)
- Merkel cells - for sensation (found in basal layer).
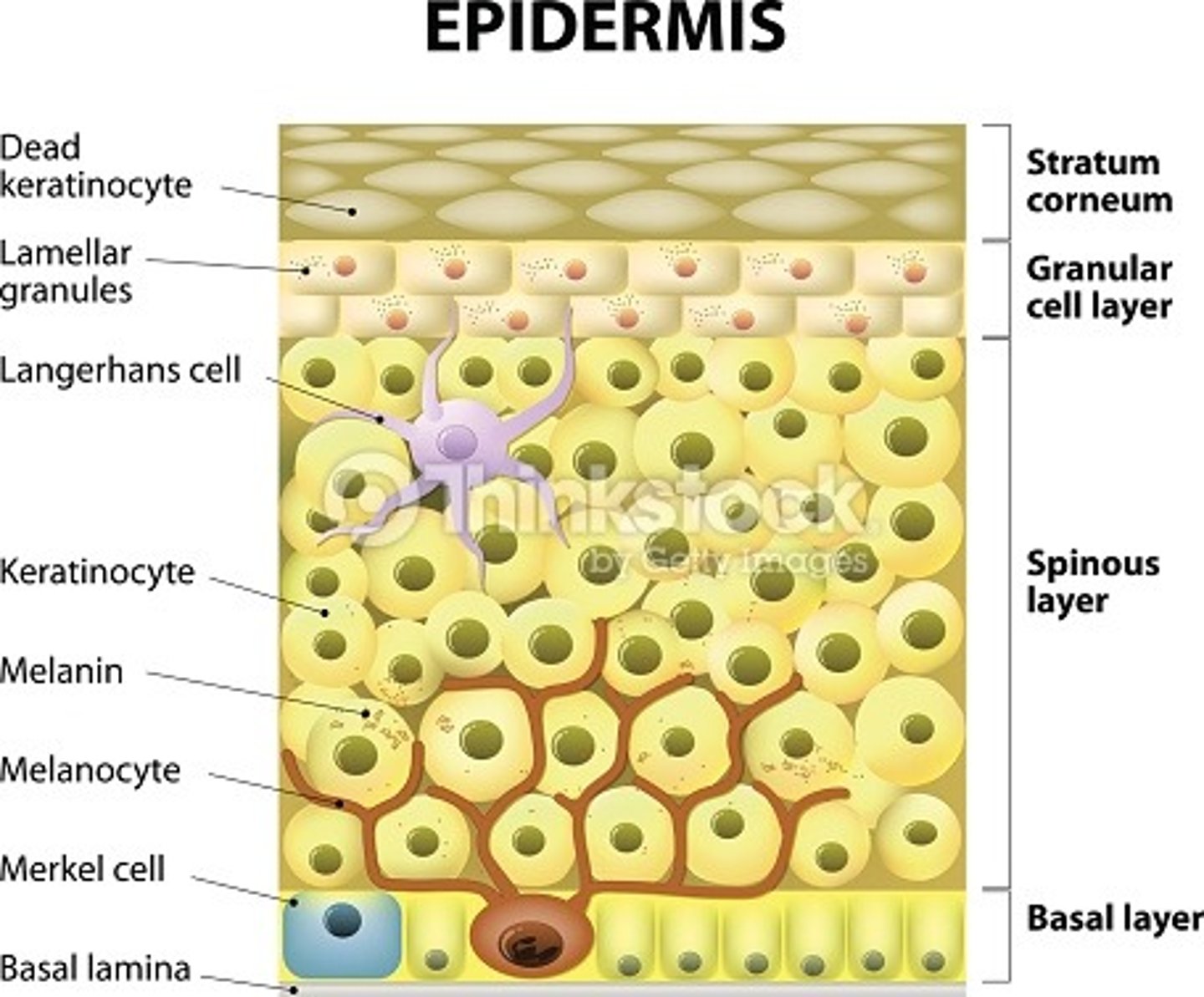
what is the most abundant cell within the epidermis of the skin?
keratinocytes
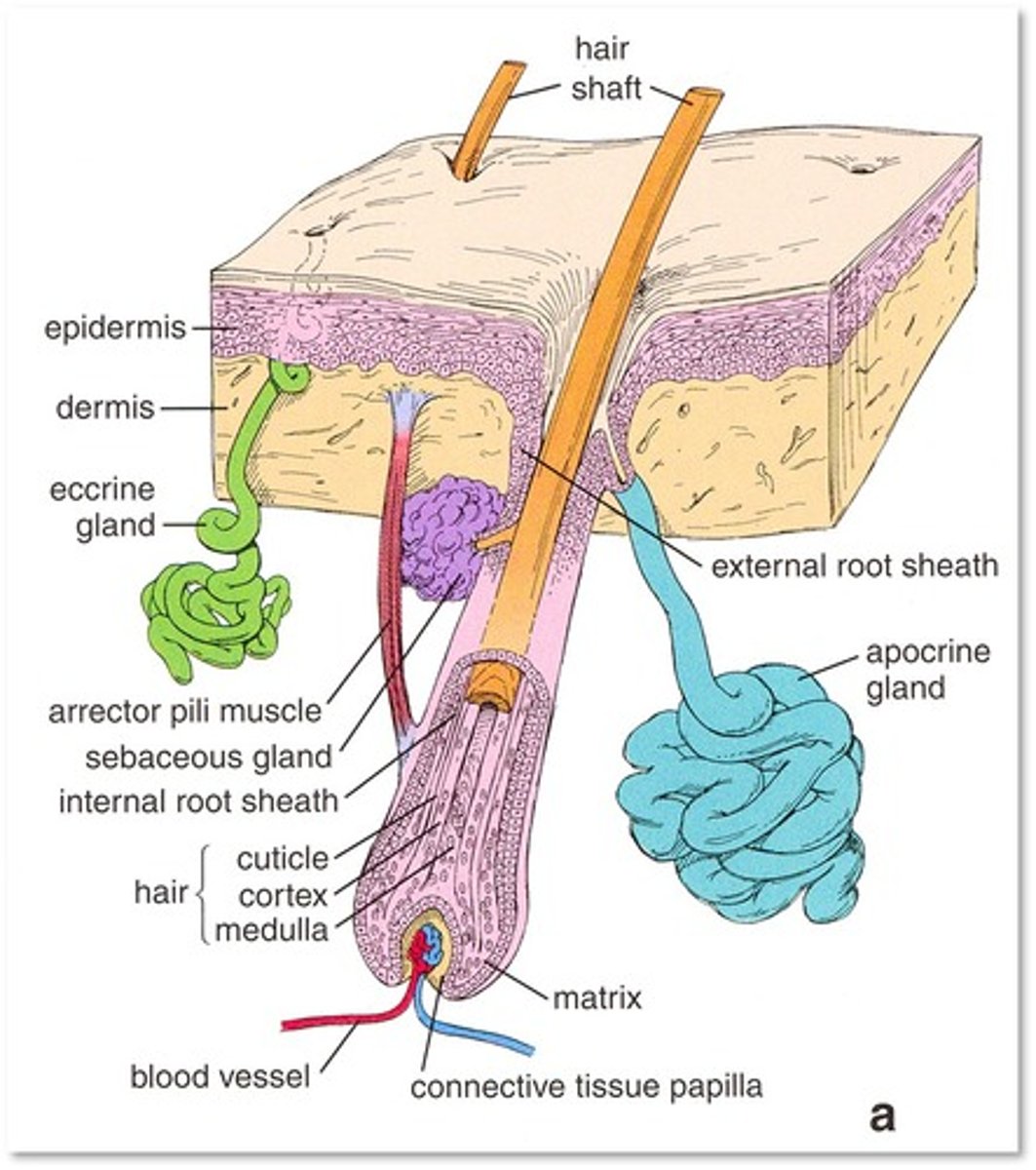
name some skin appendages present in the dermis and subcutis of the skin.
- hair follicle
- nails
- sebaceous glands (secretes sebum to coat hair)
- eccrine glands (sweat)
- apocrine glands (pheromone + body odour? but no definite function).
describe the histological layers of the dermis.
1. papillary dermis.
thin layer below dermis. loose connective tissue. fine collagen + elastin fibres to make skin stretchy. capillaries, lymphatics + nerve endings.
2. reticular dermis.
thick layer of dense irregular connective tissue with thick collagen fibres. fibroblasts, immune cells, vessels, nerves. site of most skin appendages.
name some cells of the dermis.
- connective tissue (made of collagen + elastin)
- fibroblasts (produce the collagen + elastin)
- immune cells (mast cells, macrophages, lymphocytes)
- adipocytes.
histology of the hypodermis / subcutis + ROLE
- mature adipose tissue separated by fibrous septa.
- stores energy, connects the dermis to muscles and bones, insulator, protection.
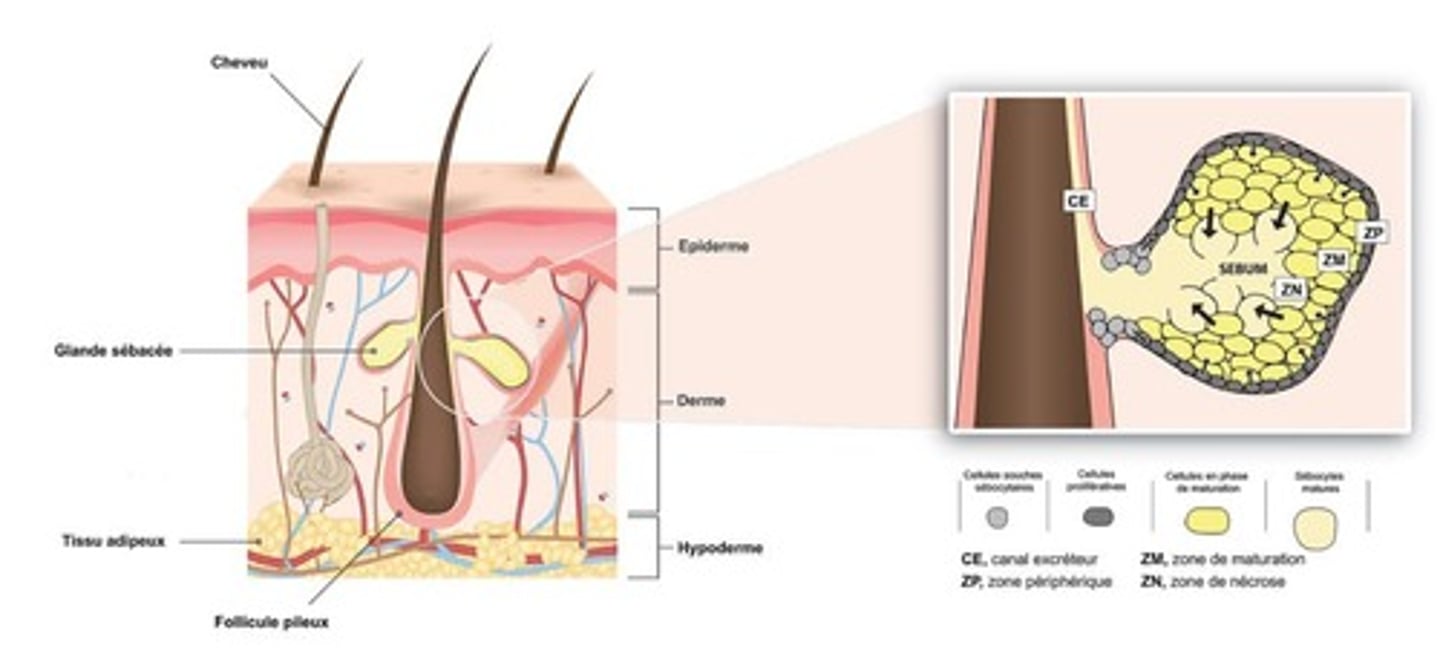
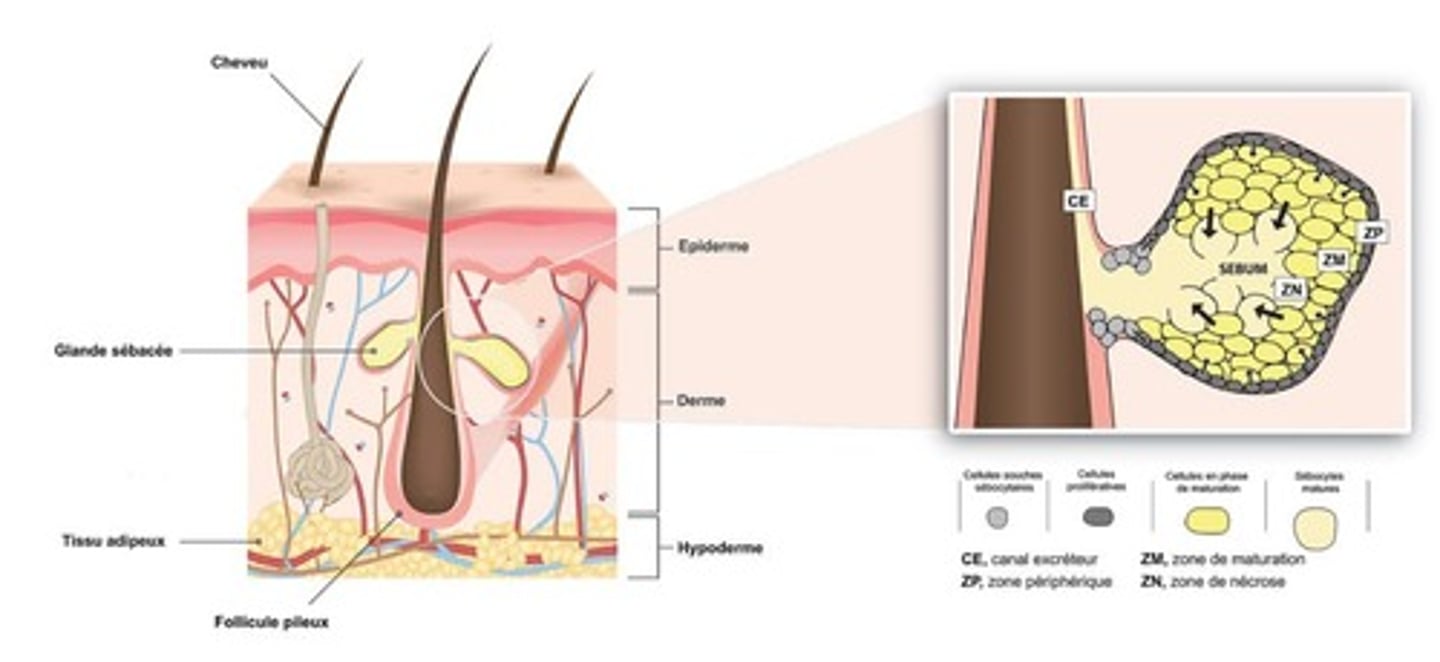
describe the structure and secretion of sebaceous glands.
structure:
- branched acinar
- the acinar are filled with vacuoles which contain a lipid. these vacuoles empty into a duct which drains into the hair follicle.
secretion:
- as the acinar is filled with lipid content (sebum), it distends and then dies, releasing the contents - this is called holocrine secretion.
describe the structure of eccrine glands and their location within the skin layers.
- coiled, tubular glands.
- secrete sweat directly onto skin via mecrocrine secretion
- location within the deep reticular dermis.
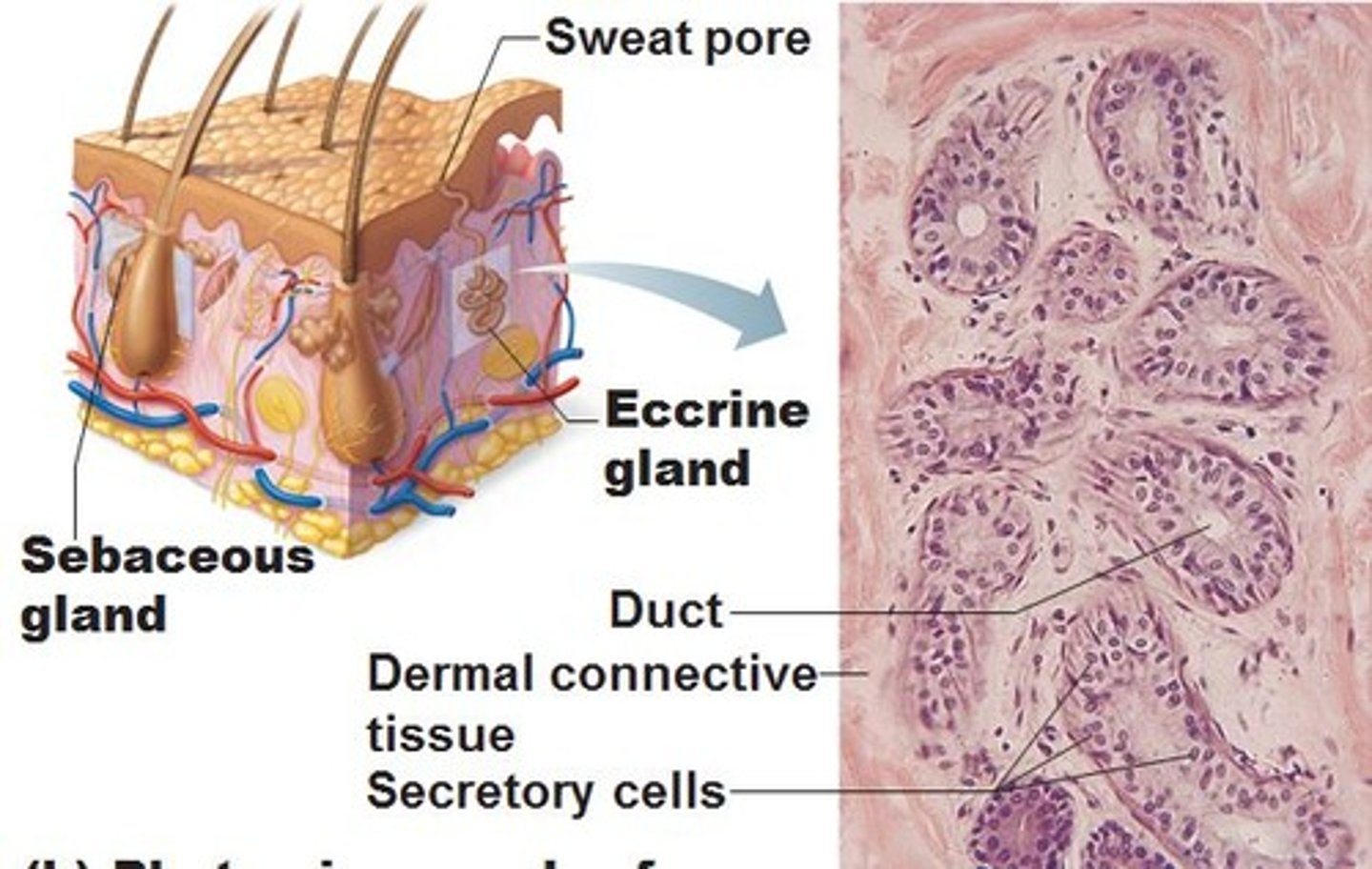
where are apocrine glands found? and describe their structure
location:
- expressed in the axillae, mammary and groin regions.
- location in the deep reticular dermis or subcutis.
structure:
- coiled, tubular glands.
- secretions are discharged into the upper part of the hair follicle.
apocrine glands only become functional during puberty.
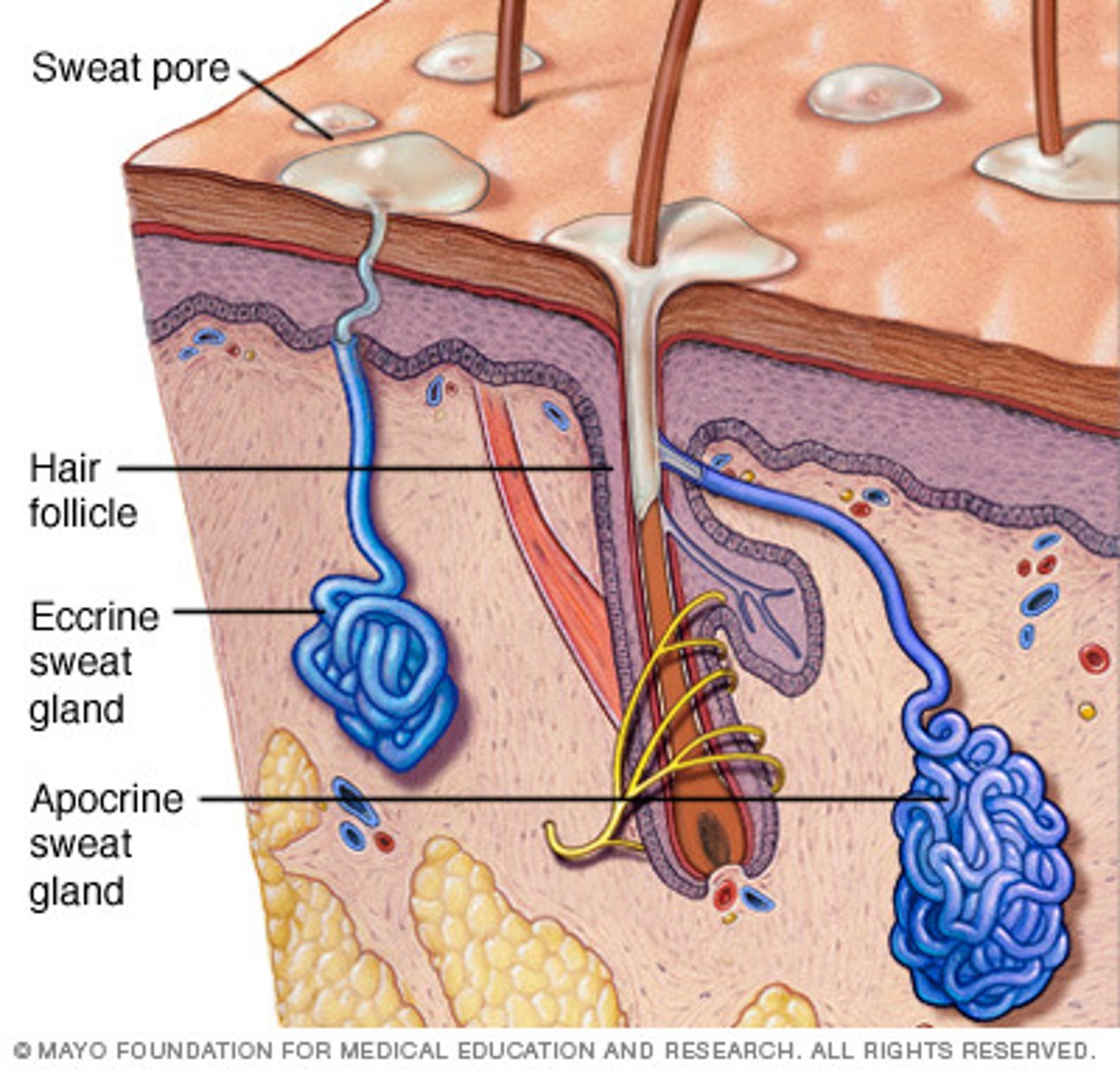
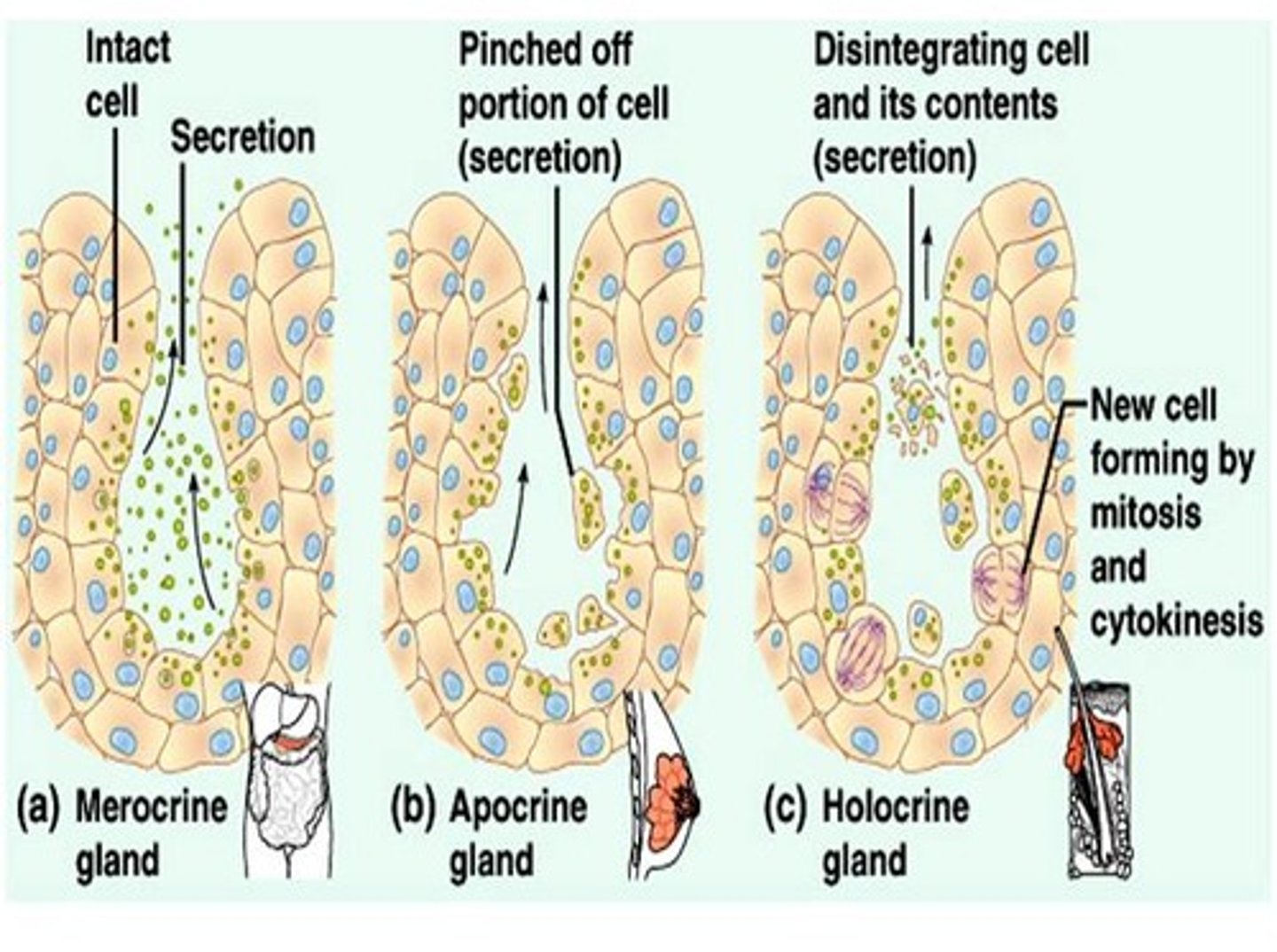
types of secretion from glands in the skin (image) + examples
1. merocrine secretion. (most abundant in body). example is eccrine gland.
2. apocrine secretion. secretes pheromones + body odour in skin. also in mammary.
3. holocrine secretion. sebaceous gland.
describe the structure of a hair shaft + production from a follicle
- produced by a hair follicle in the basal layer of the epidermis.
- at the base of the follicle is an abulbous expansion containing the hair papilla. epithelial cells in the papilla divide into the layers of organised keratin:
- central medulla, cortex and a surface cuticle.
how do superficial wounds heal?
the epidermis can regenerate from the basal layer
how to wounds extending into the dermis heal?
1. haemostasis - fibrin clot forms.
2. inflammation - to prevent infection.
3. fibroplasia - fibroblasts lay down more collagen in the CT + angiogenesis.
4. epithelialisation. migration + mitosis of keratinocytes from wound edges + remains of hair follicles form a new basal layer. then regeneration of other epidermal layers.
5. remodelling. initial new tissue is replaced to create a stronger structure.
H&E stain
Hematoxylin
- stains DNA and other acidic structures dark blue or purple Eosin
- stains other cytoplasmic components and collagen pink
Pyknosis
nuclear condensation
Karyolysis
nuclear dissolution and chromatin lysis
Karyorrhexis
nuclear fragmentation
name the three types of necrosis cell death
Coagulative, liquefactive (colliquative), and caseous (caseation)
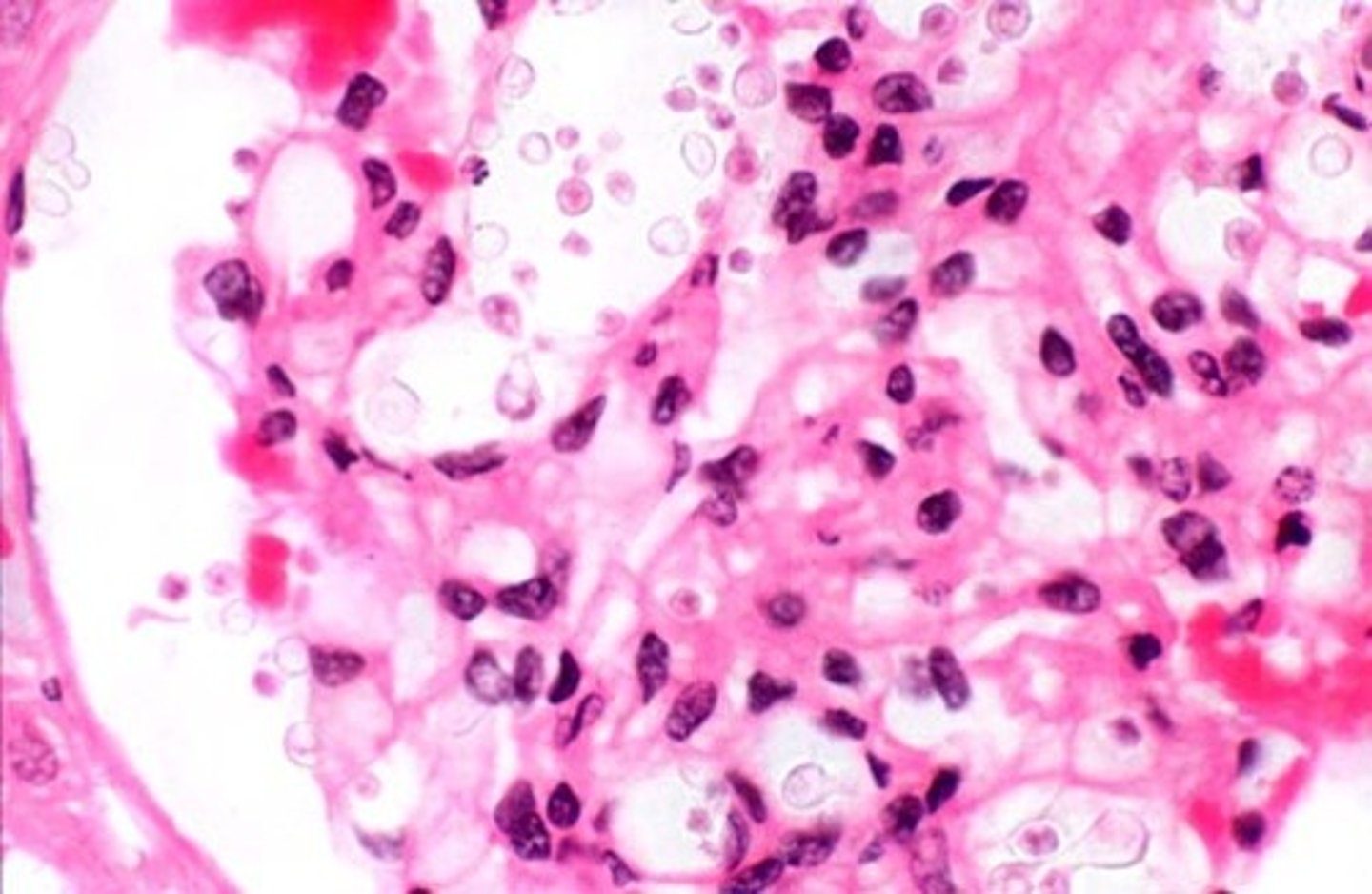
coagulative necrosis
e.g. ischemic kidney
a type of cell death that occurs when blood flow to cells stops or slows (ischemia). It can occur anywhere in the body except the brain.
appears 'ghost like' as in the tissue structure looks similar but the cells are dead.
Colliquative necrosis or liquifaction necrosis
e.g. cerebral infarct
partial or complete dissolution of dead tissue and transformation into a liquid, viscous mass.
Caseation necrosis
e.g. TB granuloma
a variant of coagulation necrosis in which dead tissue has a firm, dry, cheesy texture (dead tissue looks like cottage cheese)
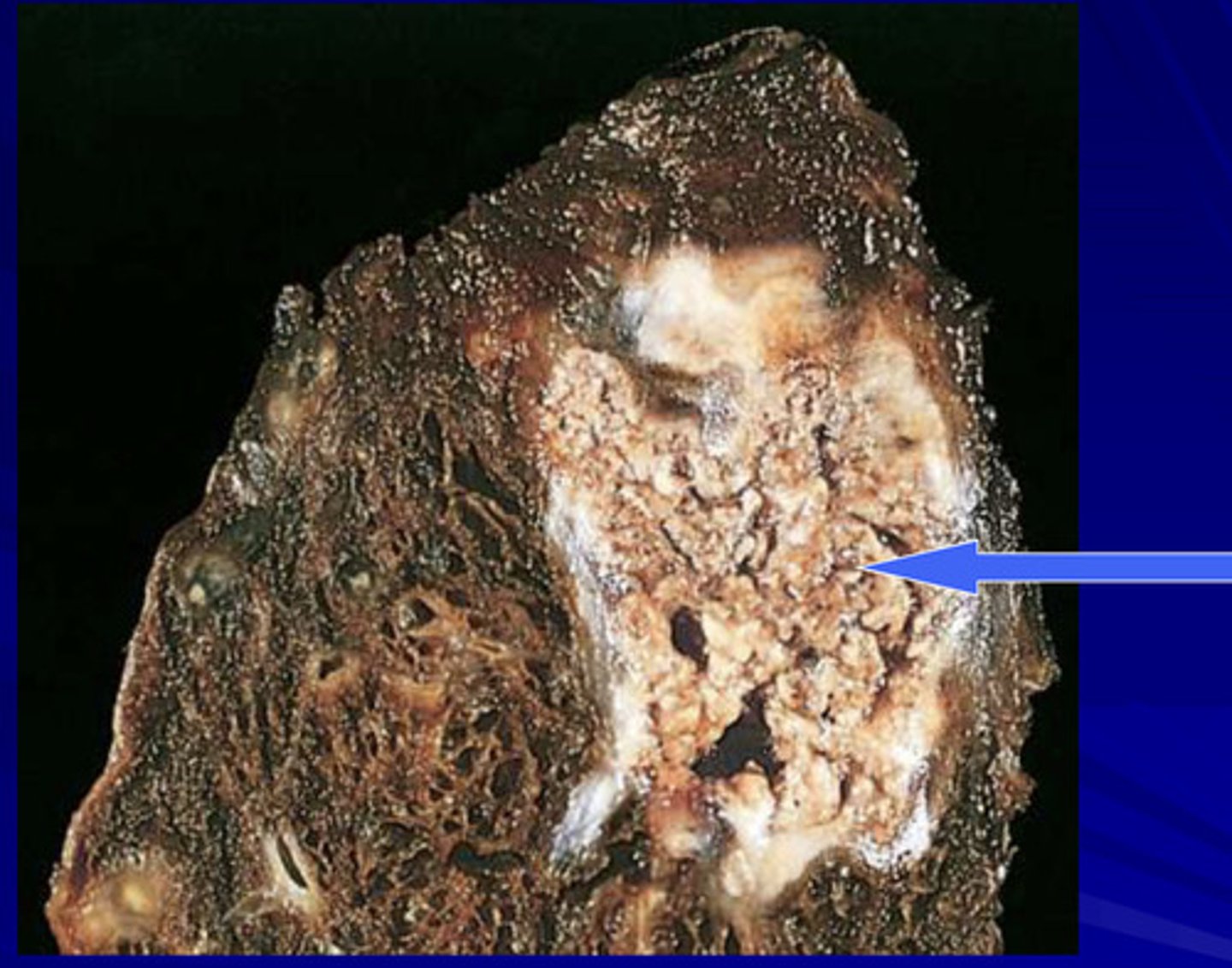
gangrenous necrosis
death of tissue from hypoxia, commonly from arteiosclerosis and affecting lower leg
may be be classified as dry, where the skin appears brown and wrinkled, or wet, where the skin appears cold, swollen and black as a result of liquefactive necrosis occurring at the site
ploidy meaning
the number of sets of chromosomes in a cell
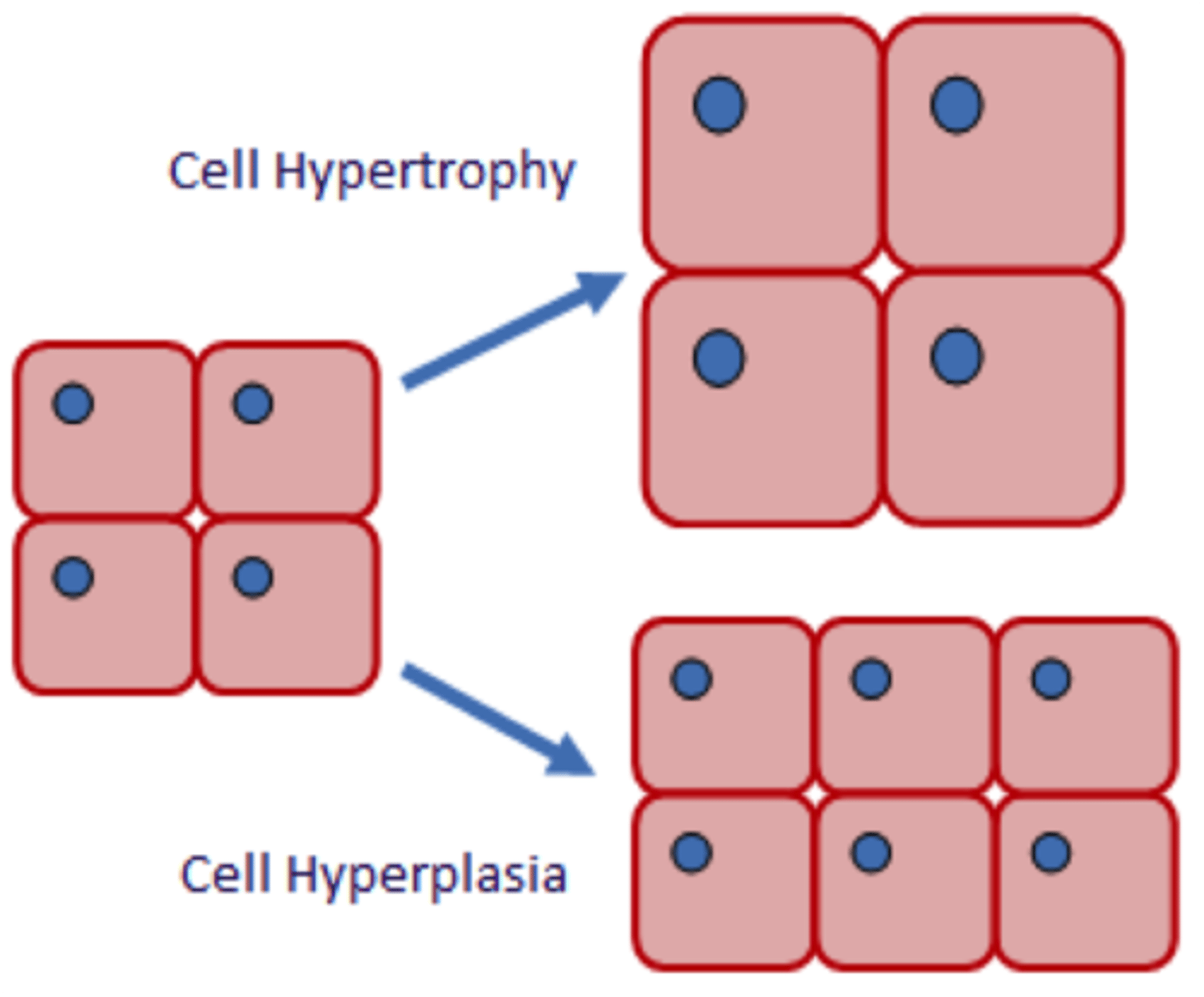
hypertrophy vs hyperplasia
Hypertrophy - increase in size
Hyperplasia - increase in number
atrophy
decrease in number and size
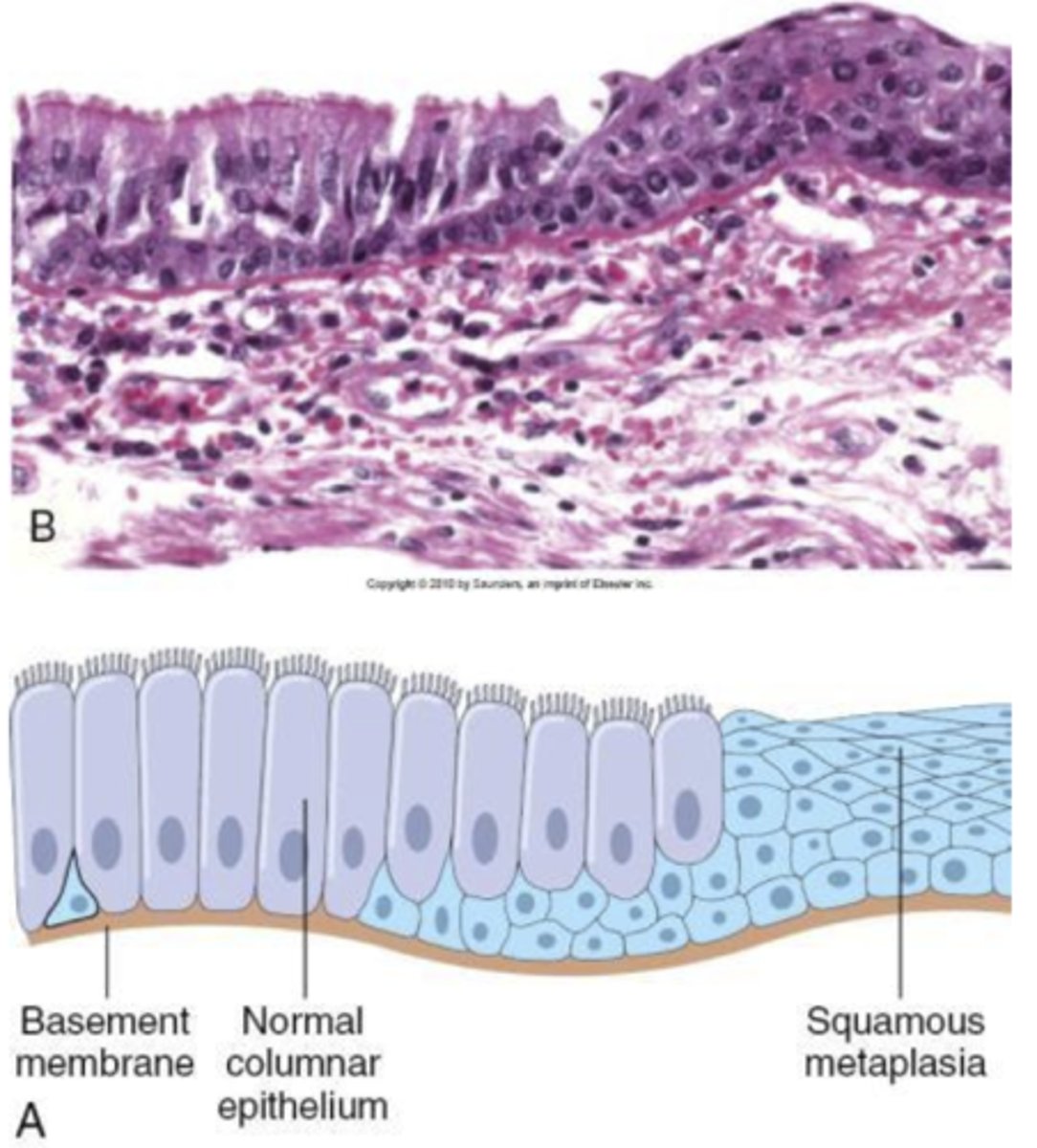
metaplasia
replacement of one differentiated cell type with another mature differentiated cell type that is not normally present in that tissue. this is different from one cell type converting into another. it is generally adaptive, benign and reversible.
persistent metaplasia can be a precursor to dysplasia which can progress to carcinoma.
dysplasia
A condition in which cells have abnormal cellular architecture, with nuclear atypia, nuclear hyperchromasia and loss of cell polarity.
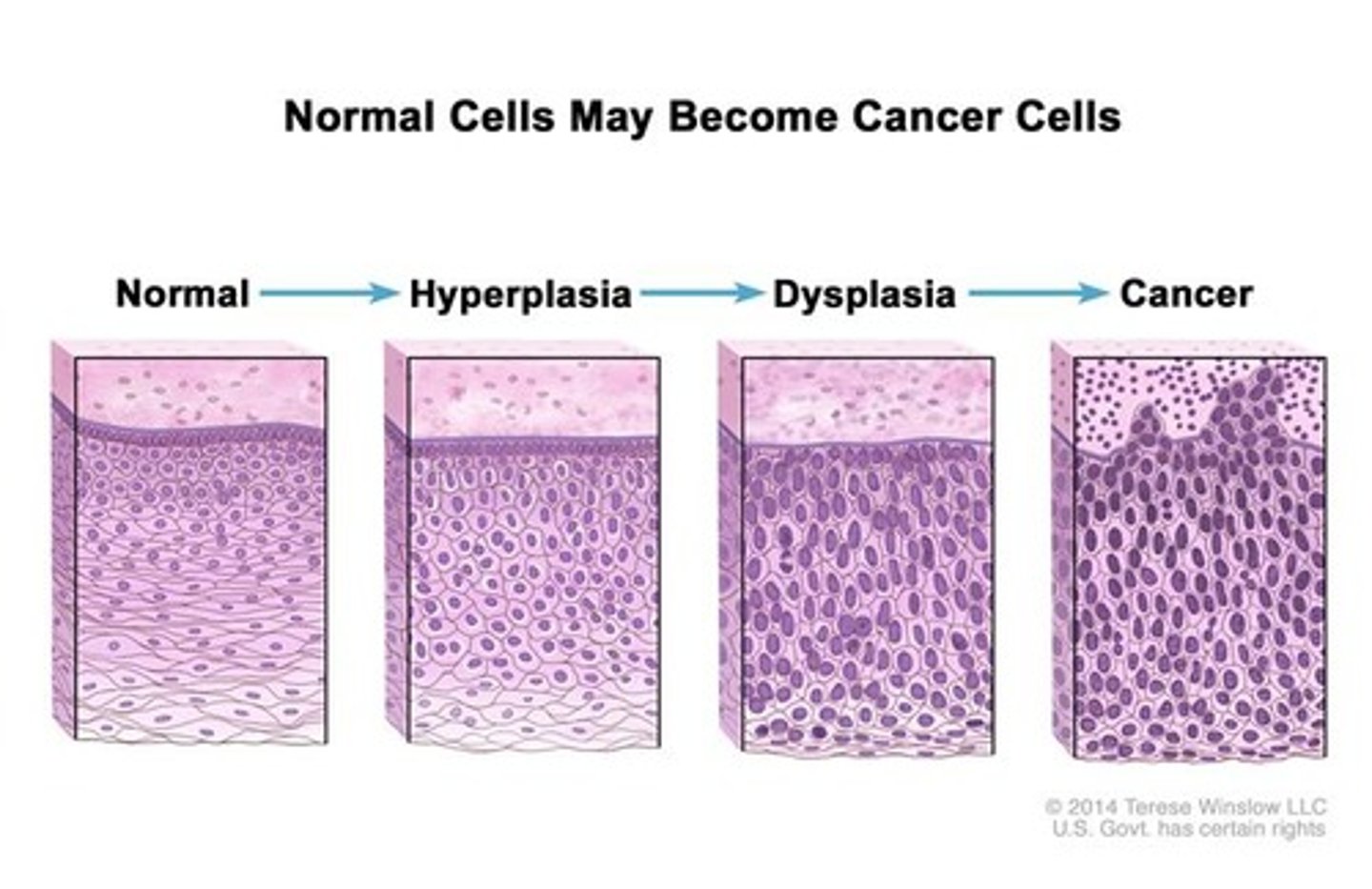
neoplasia
New, uncontrolled growth not under physiological control.
name some mechanisms underlying pathogenesis of disease
mechanical interference, competition for nutrients, tissue destruction, virulence factors, toxins, proteases, response exhaustion, self damaging (immunopathology).
iatrogenic disease
a condition that is caused by a medical treatment e.g. allergy to drug
hydrothorax
edema in the thoracic cavity
hydropericardium
edema of the pericardial cavity
what may be a cause of increased hydrostatic pressure, leading to oedema?
- generalised increased HP e.g. in heart failure
- localised to the limbs e.g. DVT
what may be a cause of decreased colloid osmotic pressure, leading to oedema?
- reduced generation of plasma proteins e.g. due to liver cirrhosis, malnutrition
- loss of proteins via skin (burns), urine (nephrotic syndrome) or faeces (in bowel disease).
what may be a cause of lymphatic obstruction, causing oedema?
- blockage by parasites
- malignancy, radiotherapy or surgery.
hyperaemia
the process by which the body adjusts blood flow to meet the metabolic needs of its different tissues in health and disease
reactive hyperaemia
local vasodilation in response to oxygen debt or accumulation of metabolic waste
active hyperaemia
increase blood flow/vasodilation in response to period of activation (increase in blood in skeletal muscles during exercise).
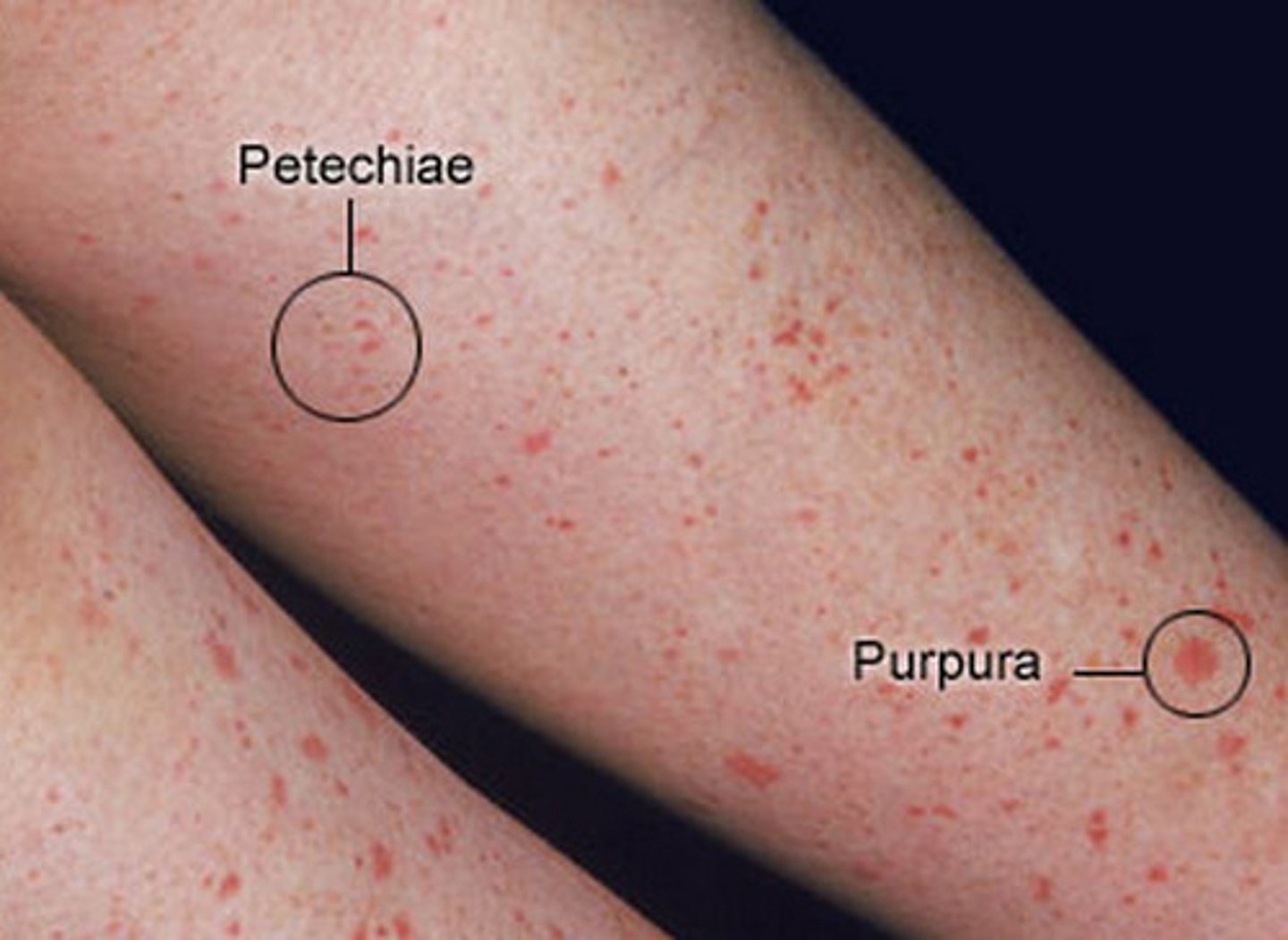
petechiae
small, pinpoint hemorrhages (1-2mm diameter)
due to thrombocytopenia, clotting factor deficiency or increased pressure in capillaries.
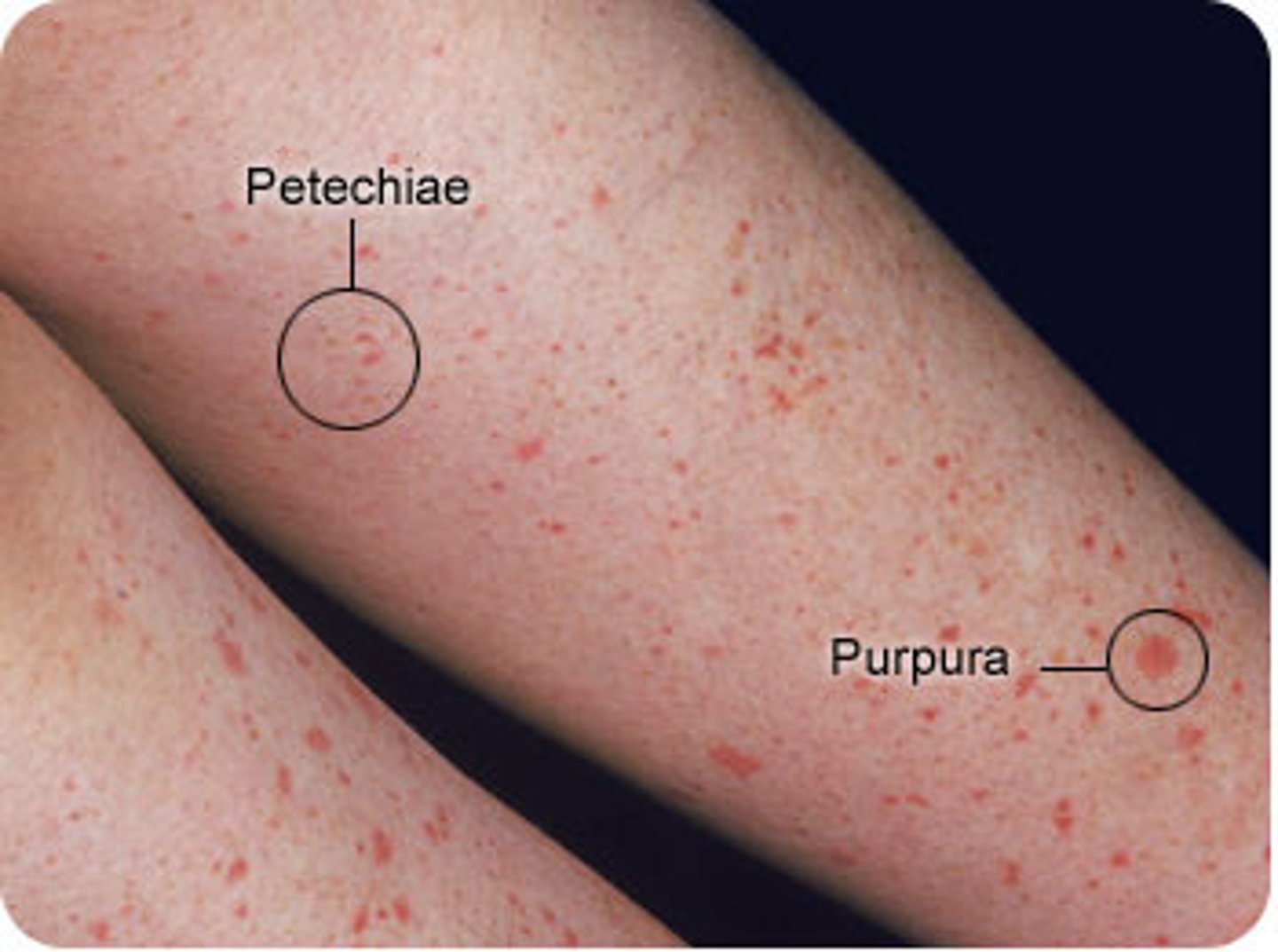
purpura
small haemorrhaging (3-10mm diameter) but bigger than petechiae
due to vasculitis or trauma.
what causes changes in the colour of a bruise?
metabolism of haemoglobin to bilirubin and hemosiderin
haematoma
a solid swelling of clotted blood within the tissues.
aka a bruise
Thrombosis vs embolism
a blood clot attached to the vessel wall vs. moving blood clot, detached from the vessel wall
types of arterial thrombi
Mural - does not occlude vessel leading to minor or transient clinical events
Occlusive - occludes the vessel leading to a major adverse clinical event.
how does the cause of arterial vs venous thrombi tend to differ?
arterial is often platelet drive whilst venous is due to changes in blood flow (stasis) and is a fibrin-driven response.
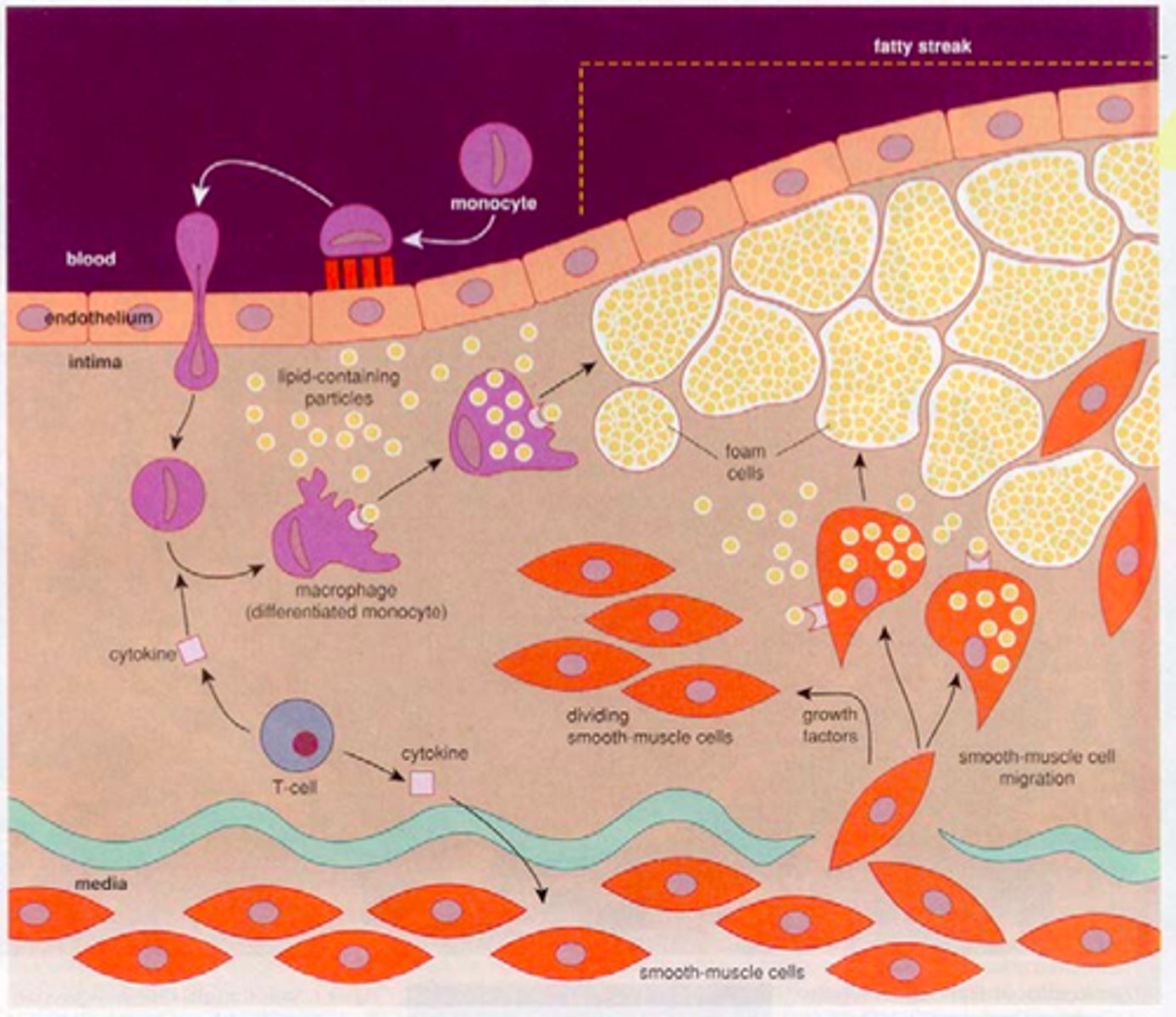
describe the stages of atheroma (atherogenesis)
1. initiation - fatty streak triggered by endothelial dysfunction. lipids (LDL) enters endothelium and forms a layer within the tunica intima. these lipoproteins are oxidised. this triggers proteins receptor expression on endothelium for leucocyte recruitment.
2. Foam cells are formed by monocytes/macrophages that have phagocytise oxidised LDLs.
2. Fibrous cap atheroma. Dead foam cells accumulate and release lipids. Lymphocytes, ECM, smooth muscle cells, collagen, elastin (all from tunica media) are encapsulated by the fibrous cap between vessel lumen and core of plaque. thin capsule is prone to rupture.
4. if the plaque ruptures, erythrocytes can accumulate and form a thrombus.
ischemia
an inadequate blood supply to an organ or part of the body.
infarction
an area of ischemic necrosis caused by occlusion of either arterial supply or venous drainage in a tissue.
this leads to an inflammatory response and scar formation.
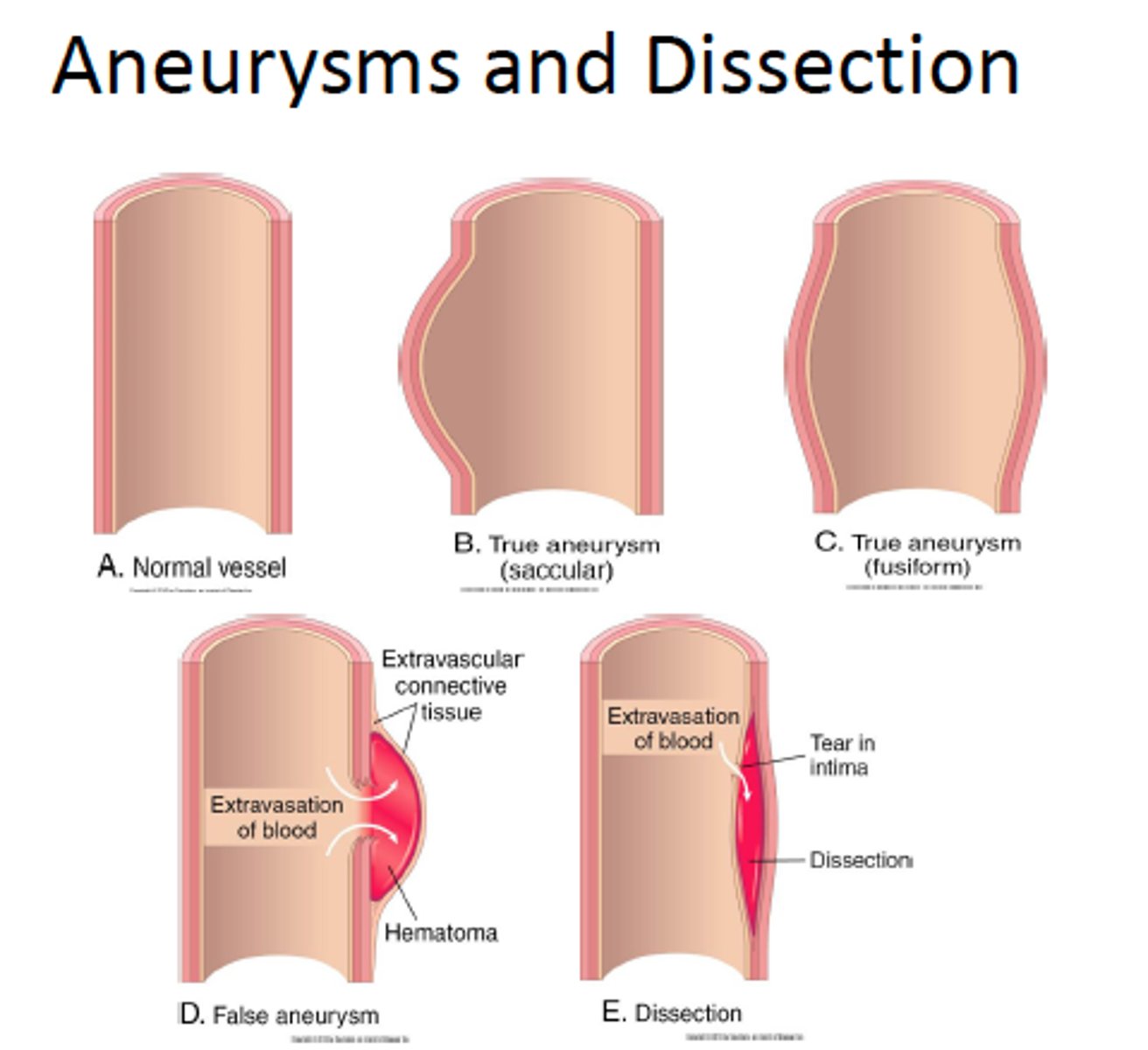
aneurysm
a localized weak spot or balloon-like enlargement of the wall of an artery
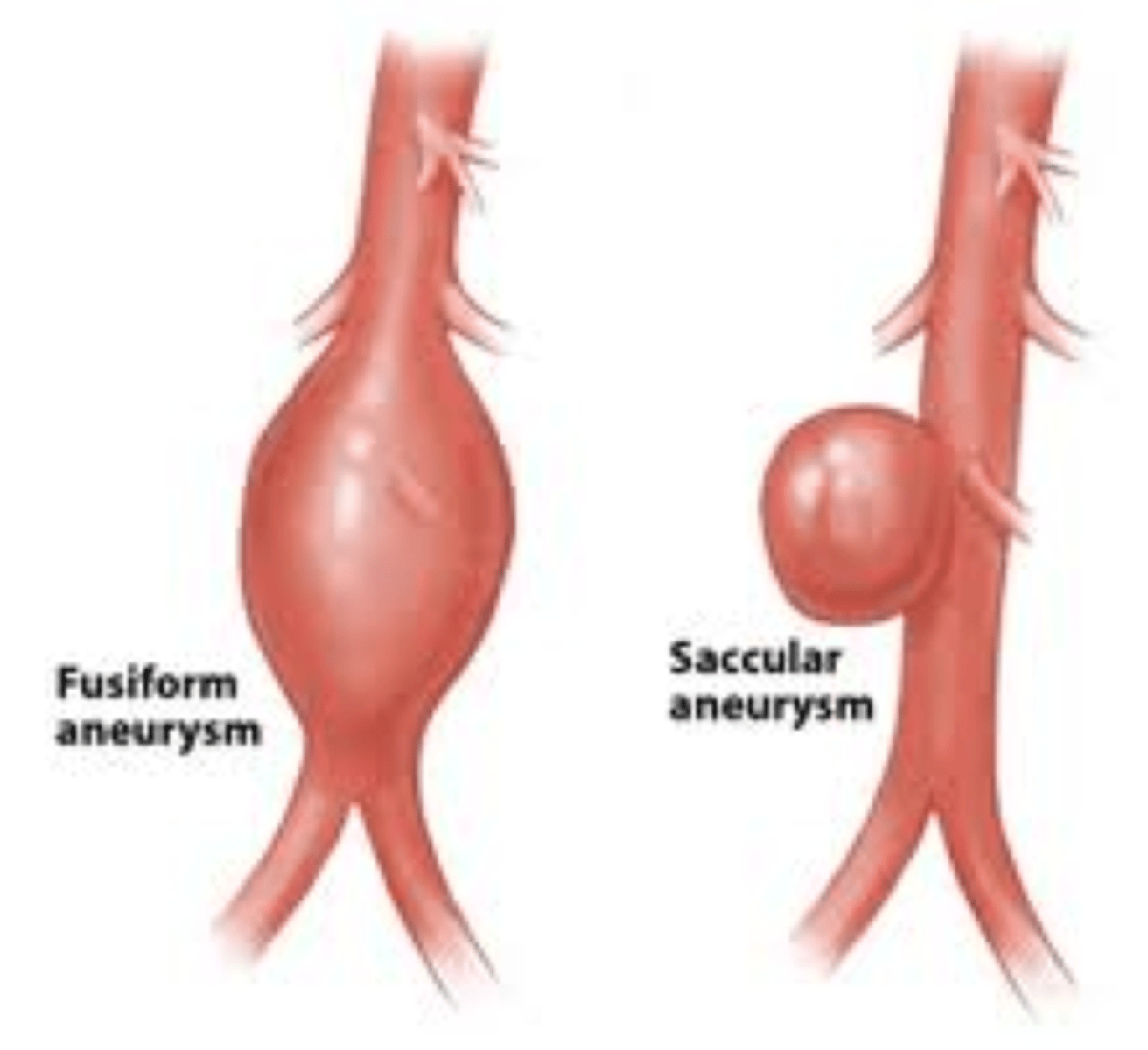
types of aneurysm by morphology
- fusiform - bulges on all sides of the blood vessel
- saccular - bulges on one side
types of aneurysm by type
- true aneurysm - involves all three layers of an artery (intimate, media and adventitia).
- false aneurysm - a locally contained hematoma. does not contain any layer of the vessel wall. a result of trauma the punctures the artery.
shock
clinical state characterised by systemic hypo perfusion (lack of blood flow) leading to reduced delivery of oxygen and nutrients.
hypoperfusion
inadequate flow of blood
types of shock
- cariogenic shock - failure of heart to pump sufficient blood.
- hypovolemic shock - loss of blood or plasma
- obstructive shock - obstruction to blood flow in the heart or pulmonary artery (e.g. in massive pulmonary embolism).
- neurogenic shock - loss of systemic blood vessel stimulation by SNS leading to vasodilation and pooling of blood in extremities and hypotension. caused by severe damage to CNS.
- septic shock - systemic infection (usually bacterial)
- anaphylactic shock
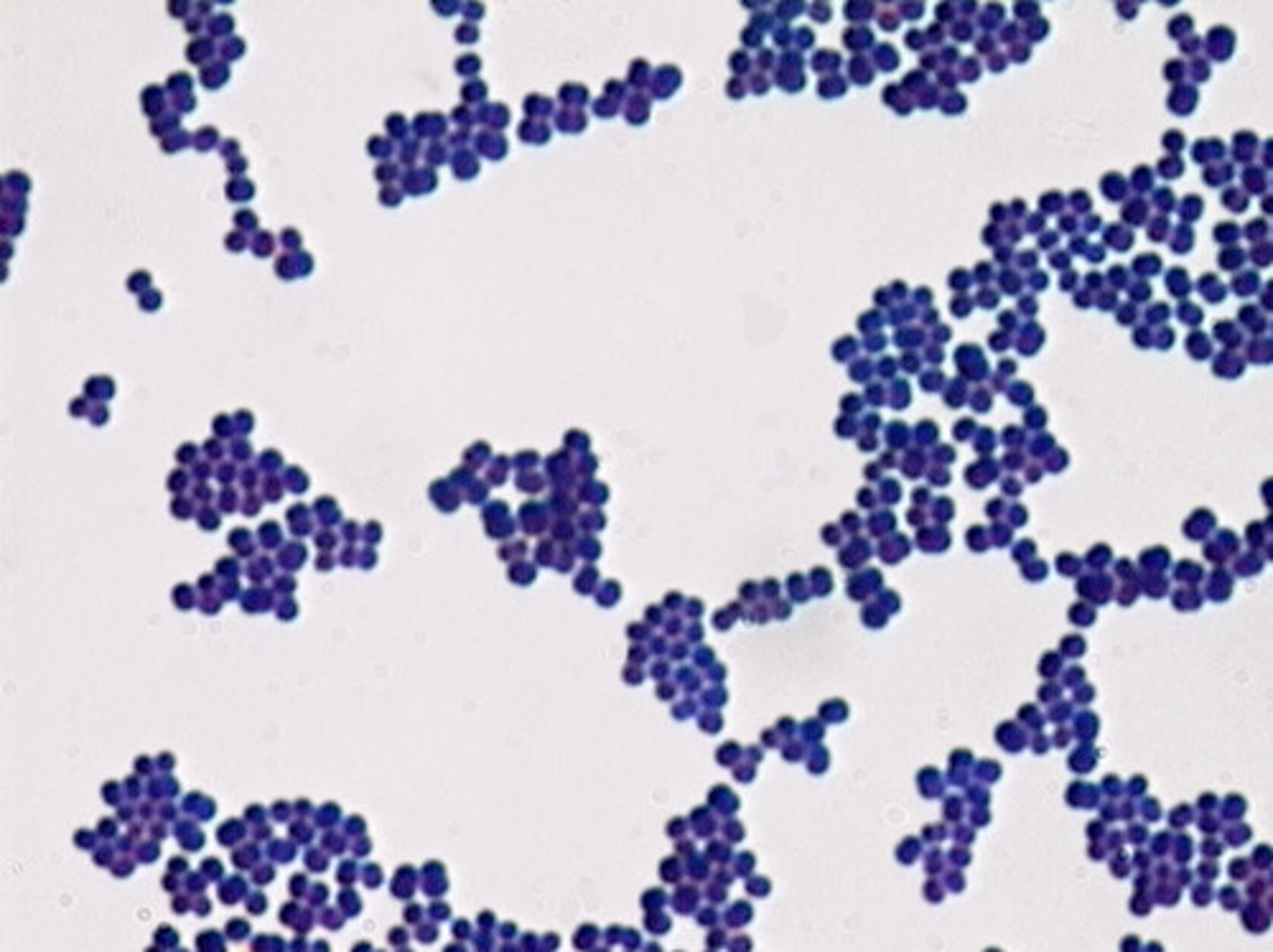
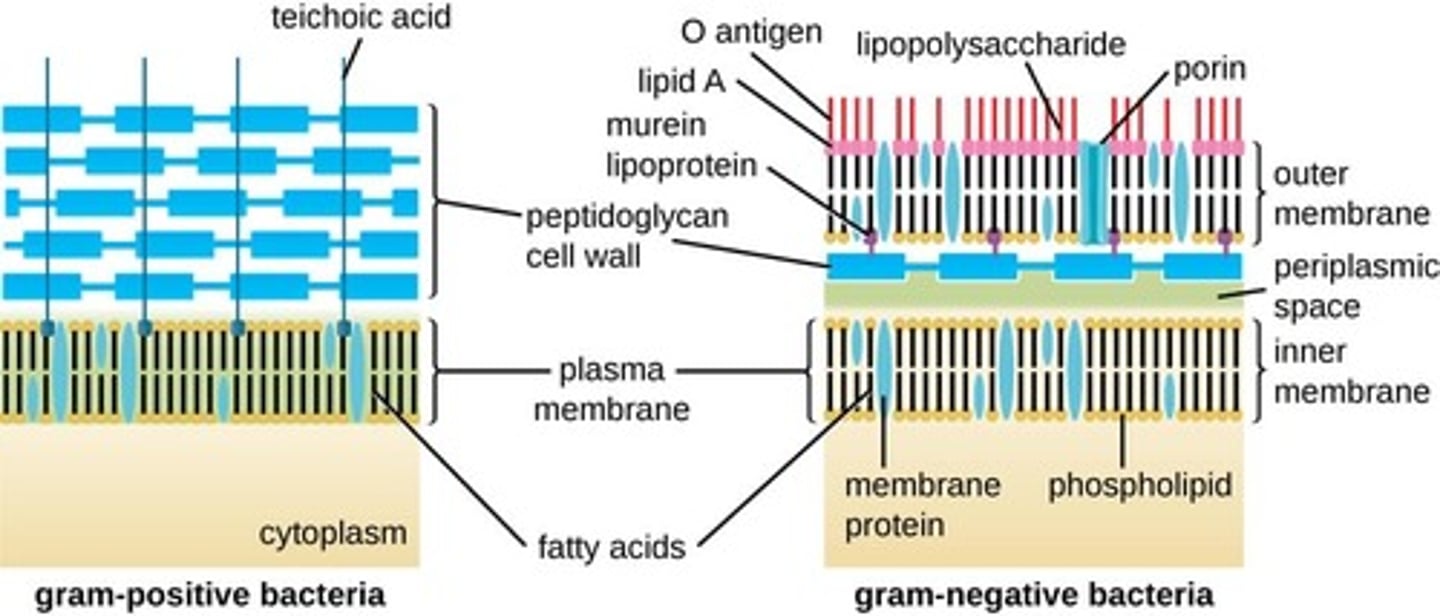
gram positive bacteria
Bacteria that have a thick peptidoglycan cell wall, and no outer LPS membrane (only a cytoplasmic membrane). They stain very darkly (purple) as the crystal violet stain is retained upon washing.
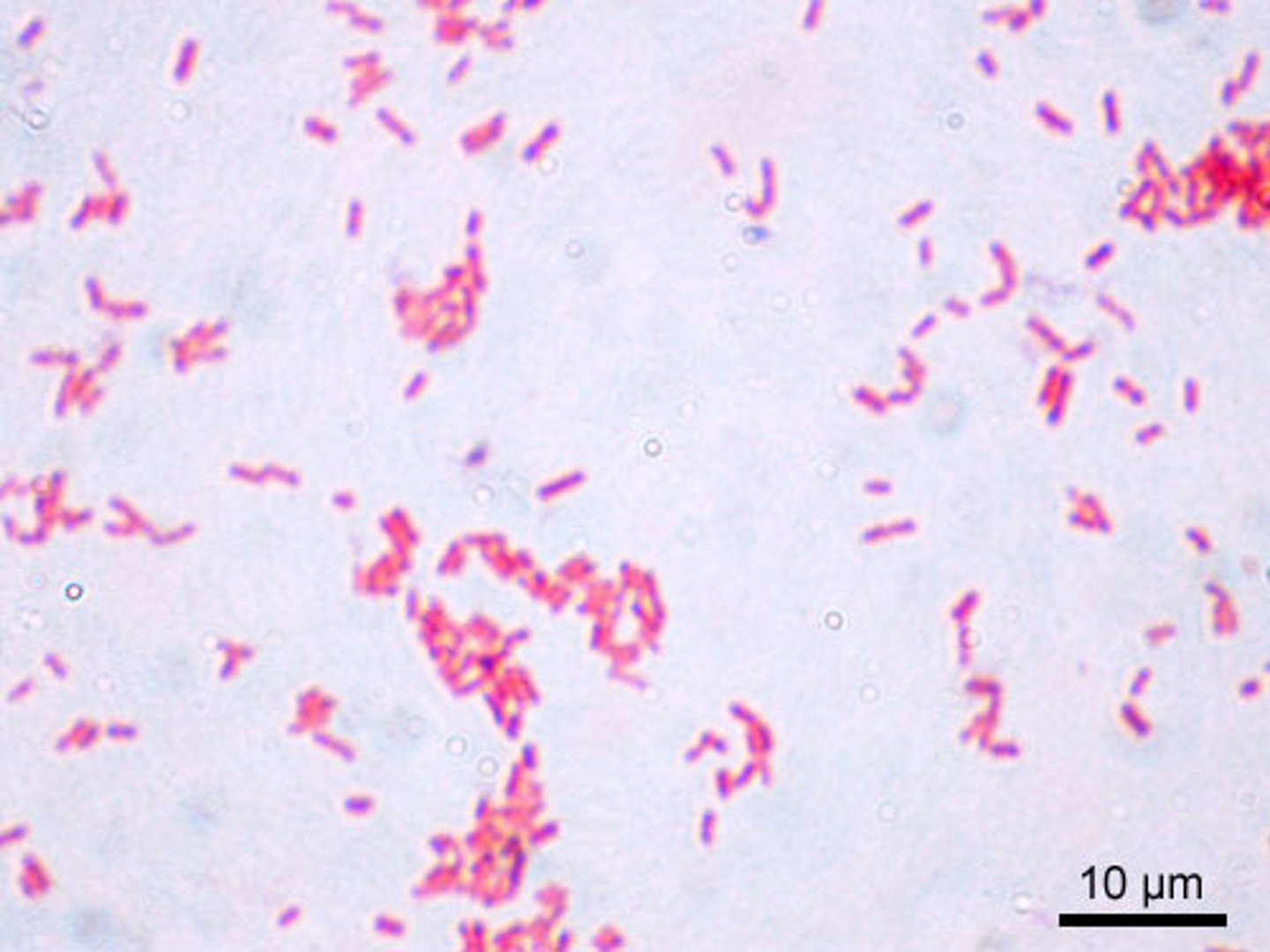
gram negative bacteria
- Bacteria that have a thin peptidoglycan cell wall covered by an outer plasma membrane (lipopolysaccharide).
- They stain pink as the crystal violet stain is washed off with alcohol and the counterstain is retained.
Gram-negative bacteria are typically more resistant to antibiotics than Gram-positive bacteria.
what is acid fast bacteria?
bacteria that consists of a cell wall made of mycolic acid. they resist decolourising by acids after staining. the final colour is pink or red.
chlamydia and TB
what is non acid fast bacteria?
bacteria that does not have a mycolic cell wall. it is readily decolourised by acids after staining. final colour is blue.
which type of bacteria (gram + or -) are able to form spores?
gram positive
by what methods can bacterial DNA be exchanged horizontally?
1. transformation - free DNA is taken up.
2. transduction - dna transferred between bacteria via a virus.
3. conjugation - plasmids being transferred.
4. transposition - extracting small parts of the bacterial dna to become the bacterial plasmid or reintegrating this part of bacterial plasmid back into the bacterial chromosome
commensal bacteria
bacteria living in or on our body without causing harm
opposite of pathogenic
overt or strict bacteria
only associated with human disease and are not found on members of the normal, healthy microbial flora
Facultative bacteria
bacteria that can survive and grow in environment as well as a host. (accidental host).
name some bacterial virulence factors that facilitate colonisation of a host
- adhesins (fimbriae, pili, outer membrane protein)
- flagella (motility, penetrate mucin in the gut)
- factors to obtain essential nutrients
- toxins
- capsule (prevents phagocytosis)
- type III secreted molecules (bacterium secreted directly into host cell)
endotoxins
the lipopolysaccharide components of the outer membrane of gram-negative bacteria
weakly toxic
exotoxins
proteins released extracellularly by certain gram positive and negative bacteria
usually highly toxic with a specific target
enterotoxin
a subgroup of exotoxins that act on the small intestine. they can change the intestinal permeability leading to diarrhoea
toxoid
inactivated toxin used in a vaccine
describe the structure of bacteria
coccus
spherical
bacillus
rod shaped
fastidious bacteria
Bacteria which have complex and specific nutritional requirements for growth and survival.
which layers of the skin are affected by a first degree burn?
only the epidermis
which layers of the skin are affected by a second degree burn?
- 2nd degree superficial partial thickness burn = epidermis and papillary layer of dermis
- 2nd degree deep partial thickness burn = epidermis and most of papillary and reticular dermis
which layers of the skin are affected by a third degree burn?
all of epidermis and dermis
which layers of the skin are affected by a fourth degree burn?
epidermis, dermis and some/all of the hypodermis
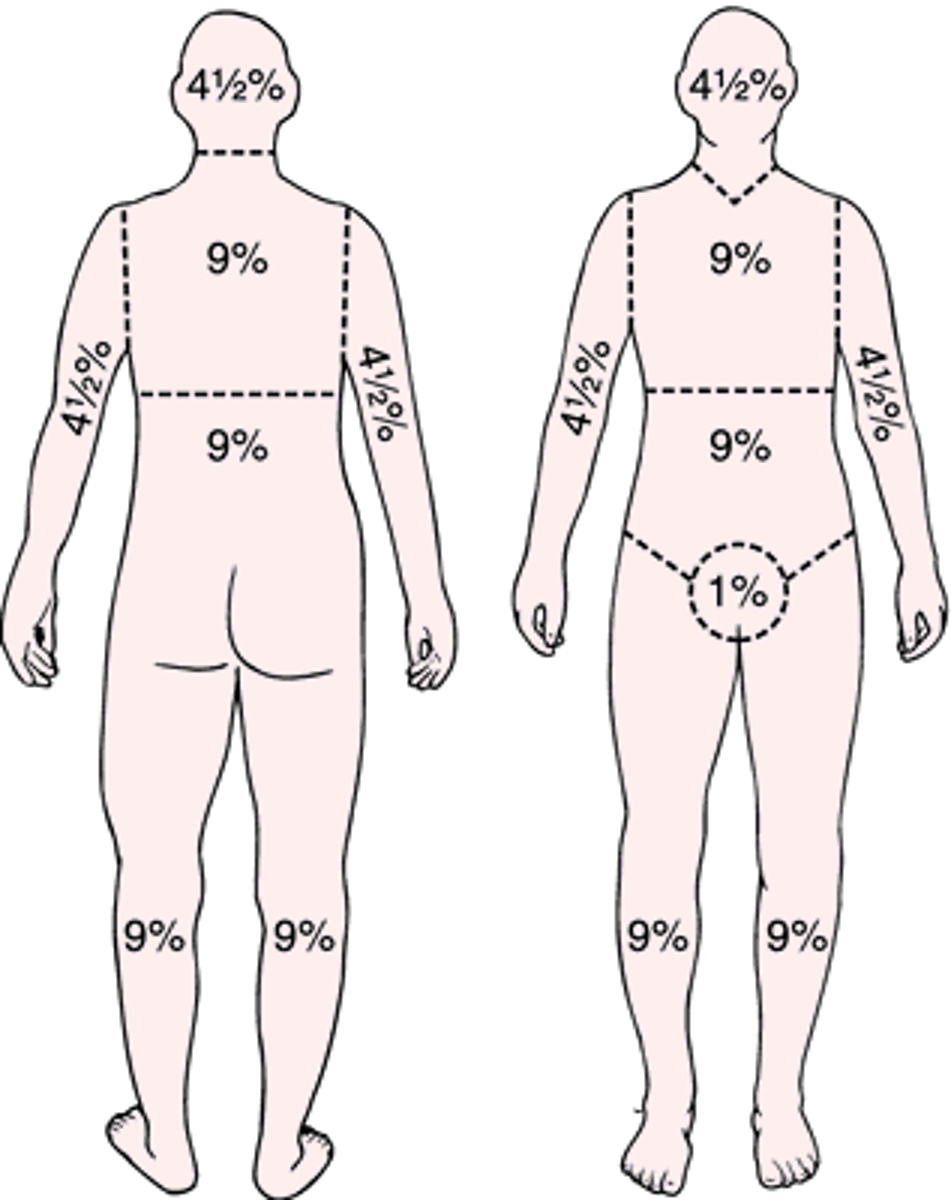
how is % of body burned calculated?
rule of nines
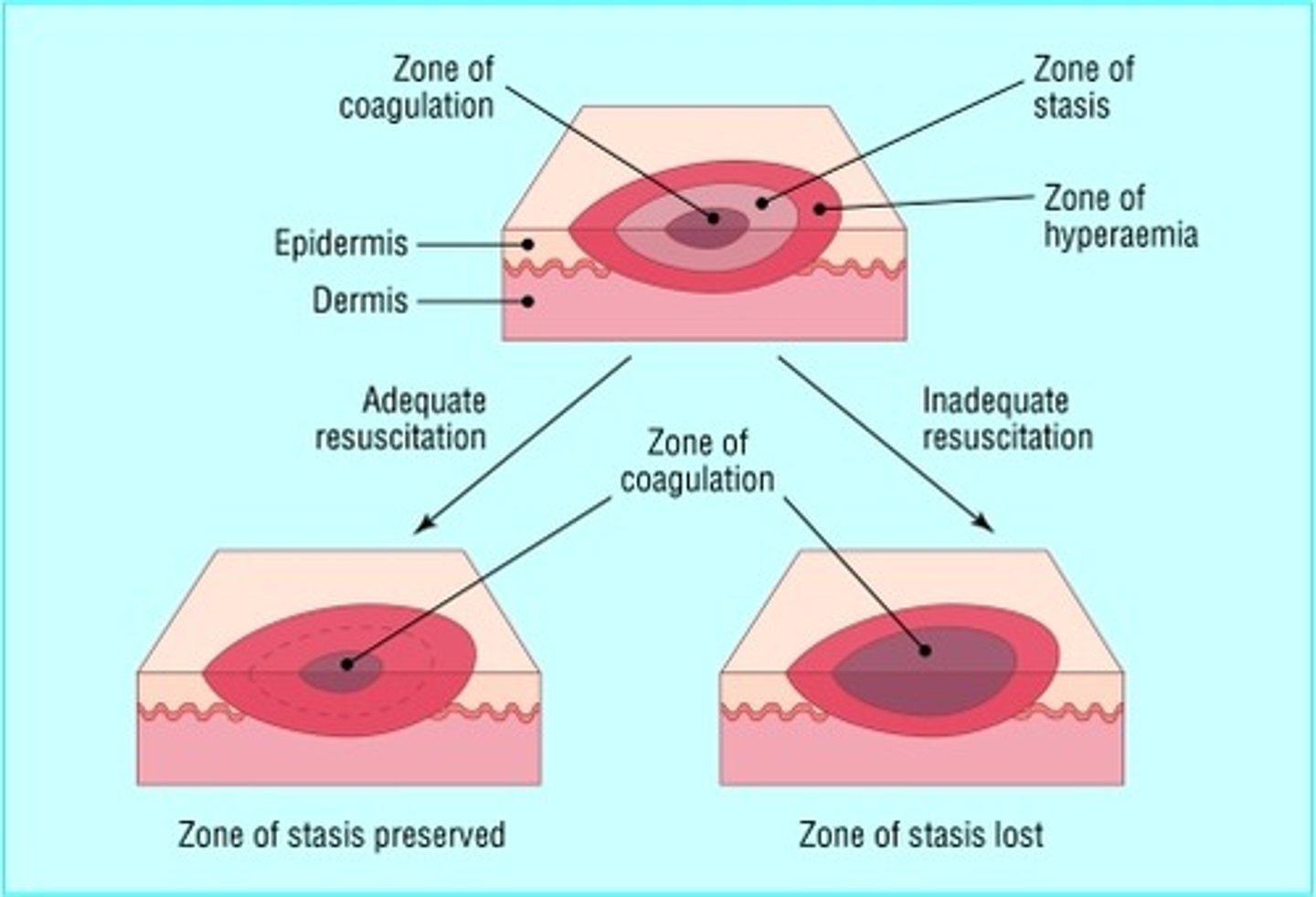
zone of burns
Zone of coagulation—This occurs at the point of maximum damage. In this zone there is irreversible tissue loss due to coagulation of the constituent proteins.
Zone of stasis— decreased tissue perfusion, potentially salvageable tissue.
Zone of hyperaemia— outermost zone. tissue perfusion is increased. The tissue here will invariably recover unless there is severe sepsis or prolonged hypoperfusion.
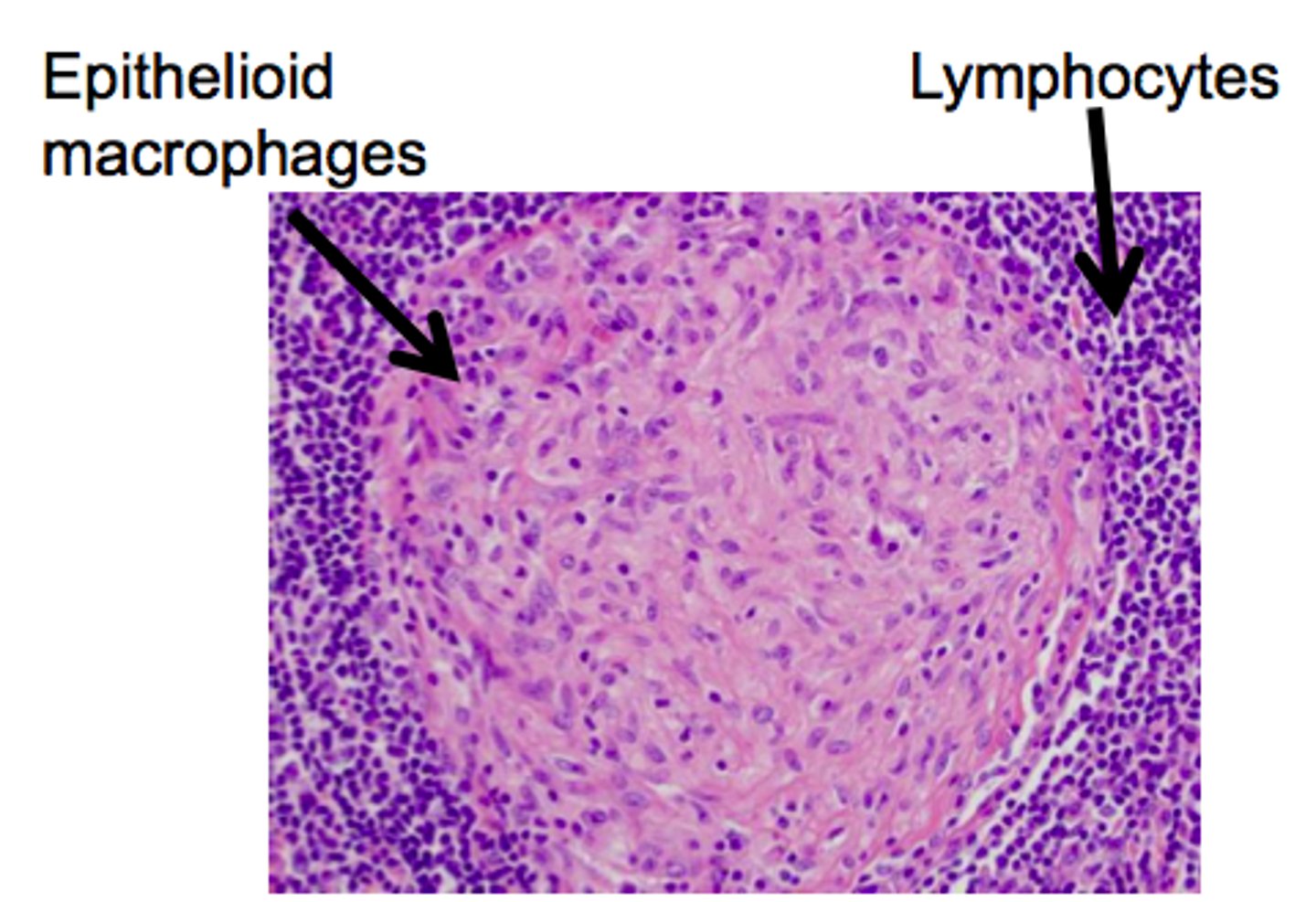
Granulomatous inflammation
- type of chronic inflammation due to the presence of granulomata
- this granuloma is a collection of macrophages surrounded by a ring of lymphocytes
- these macrophages are larger than normal and have a pink cytoplasm (they are called epithelioid cells)
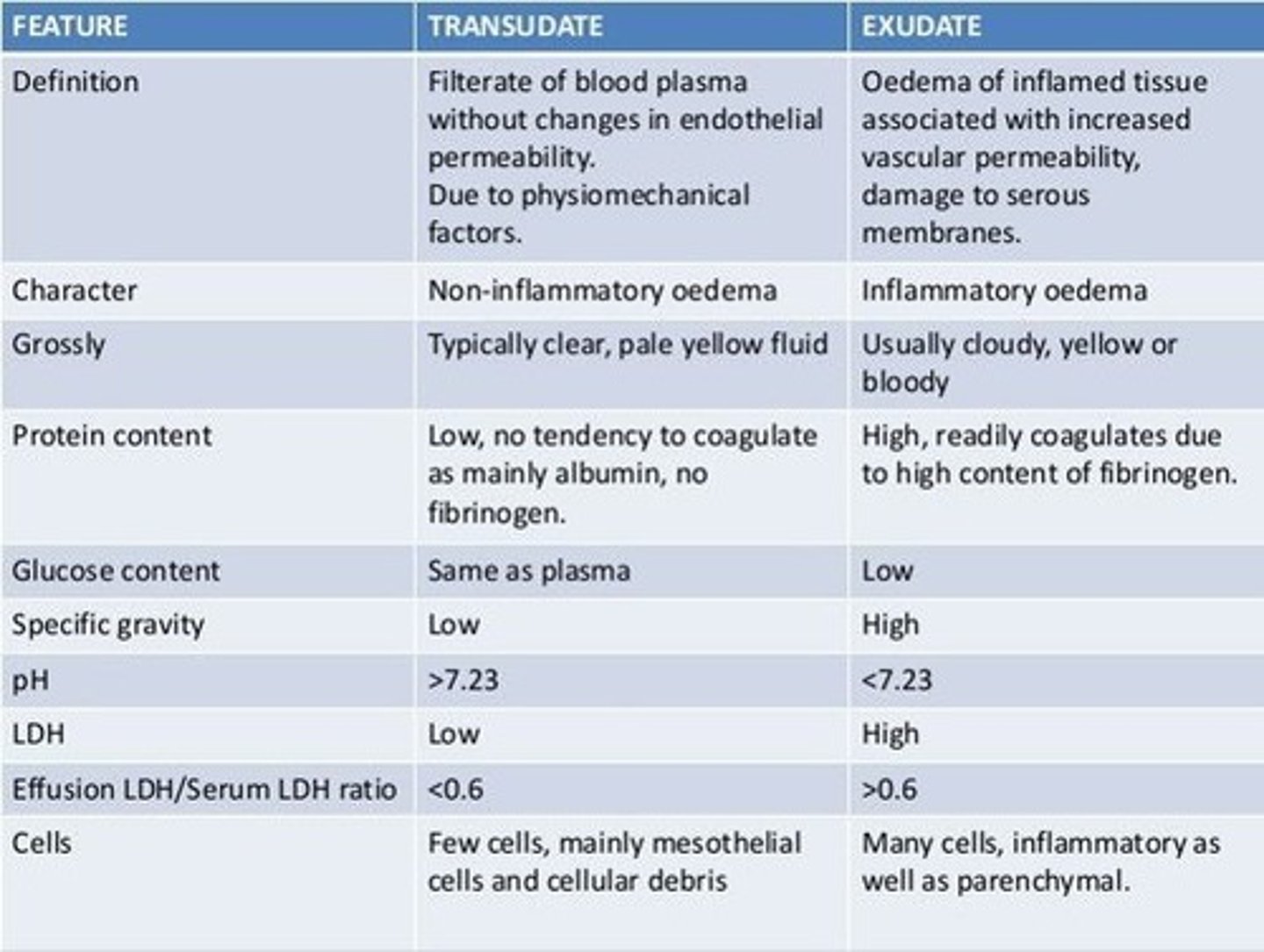
Transudate
noninflammatory fluid that resembles serum but with slightly less protein
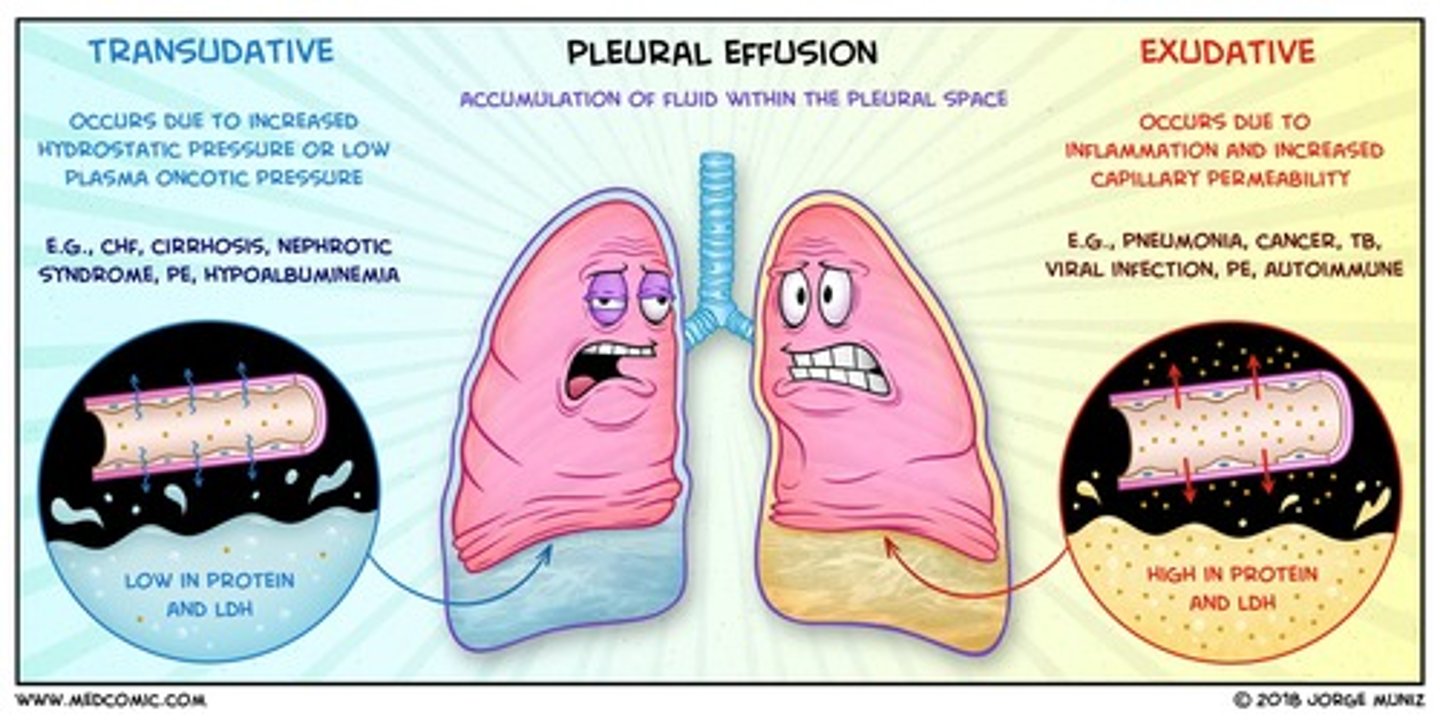
Exudate
- aka pus
- Fluid that leaks out of blood vessels into nearby tissues.
- Made of cells, proteins, and solid materials.
- Oozes from cuts or from areas of infection or inflammation.
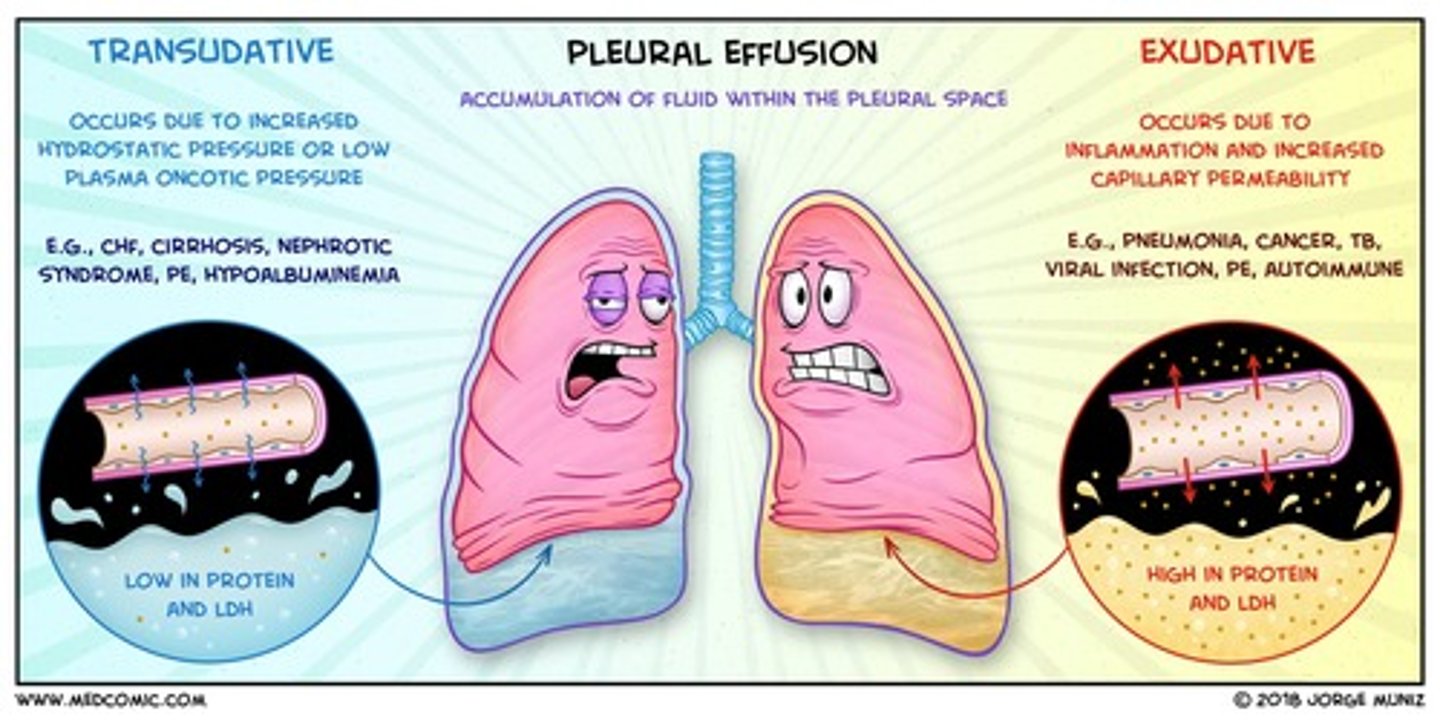
compare the components of transudate vs exudate
- exudate is higher in protein content than transudate
- exudate has more white blood cells than transudate
- exudate clots more easily as high fibrinogen content
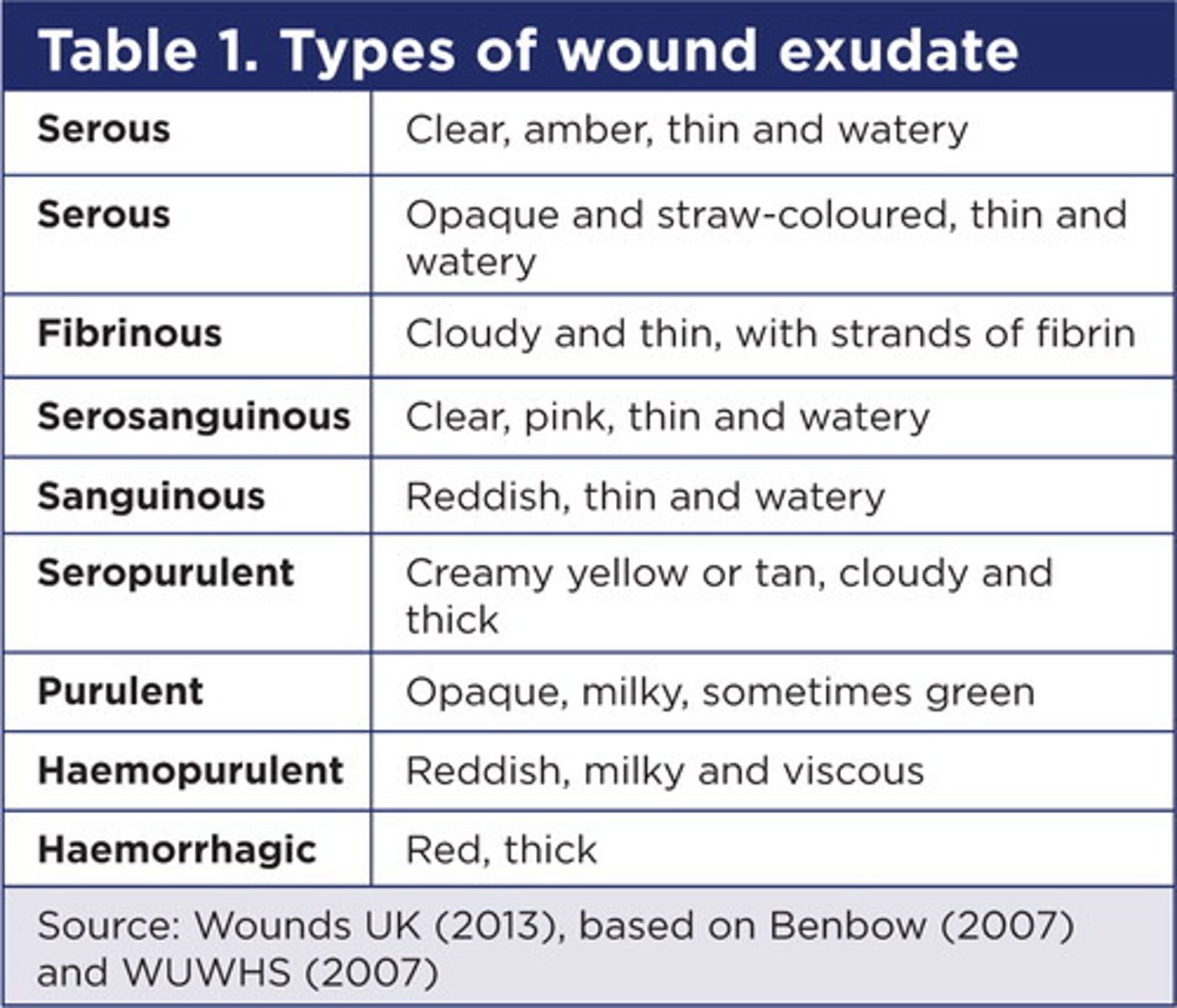
what are the types of exudate?
- Serous: it appears thin, watery, and straw-colored. It is normal and most common.
- Fibrinous: it is thin, watery, and cloudy in appearance.
- Sanguineous: bloody drainage
- Serosanguinous: clear but blood-tinged drainage
- Purulent - a thick yellow, brown, green or grey drainage
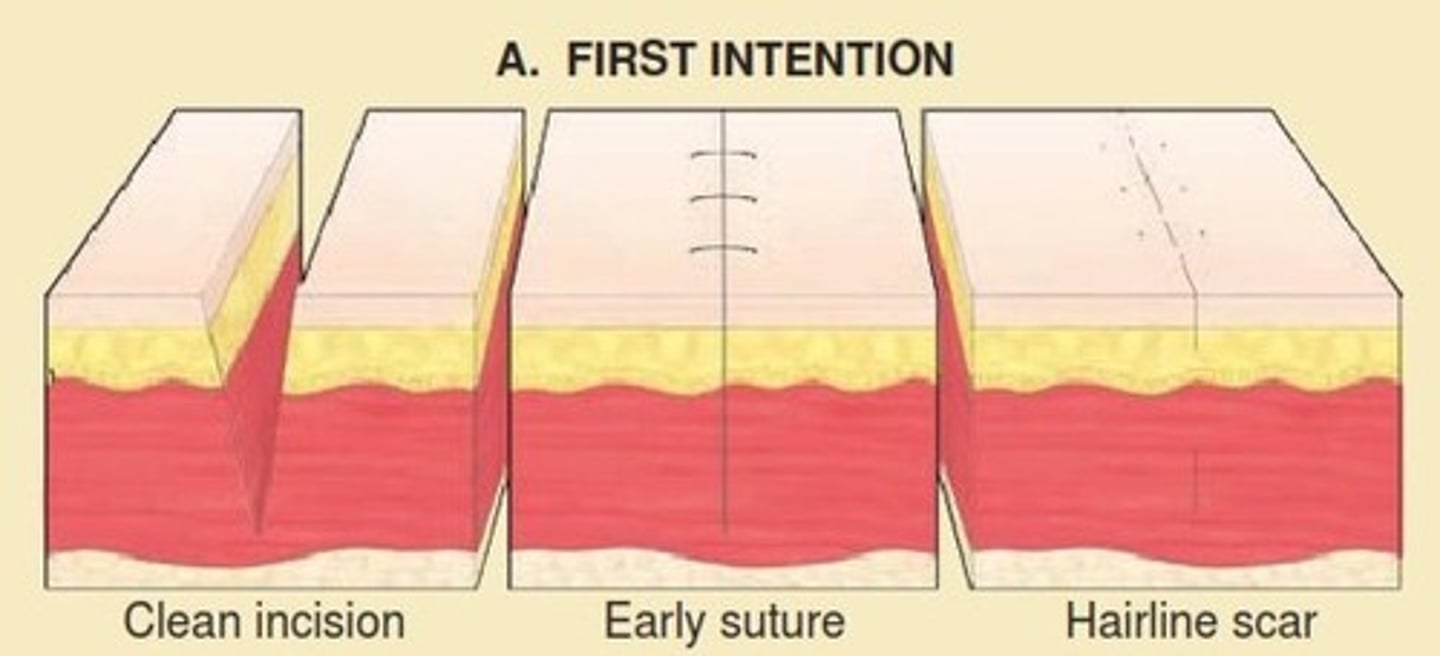
Healing by primary intention
Healing of a sharp and clean wound.
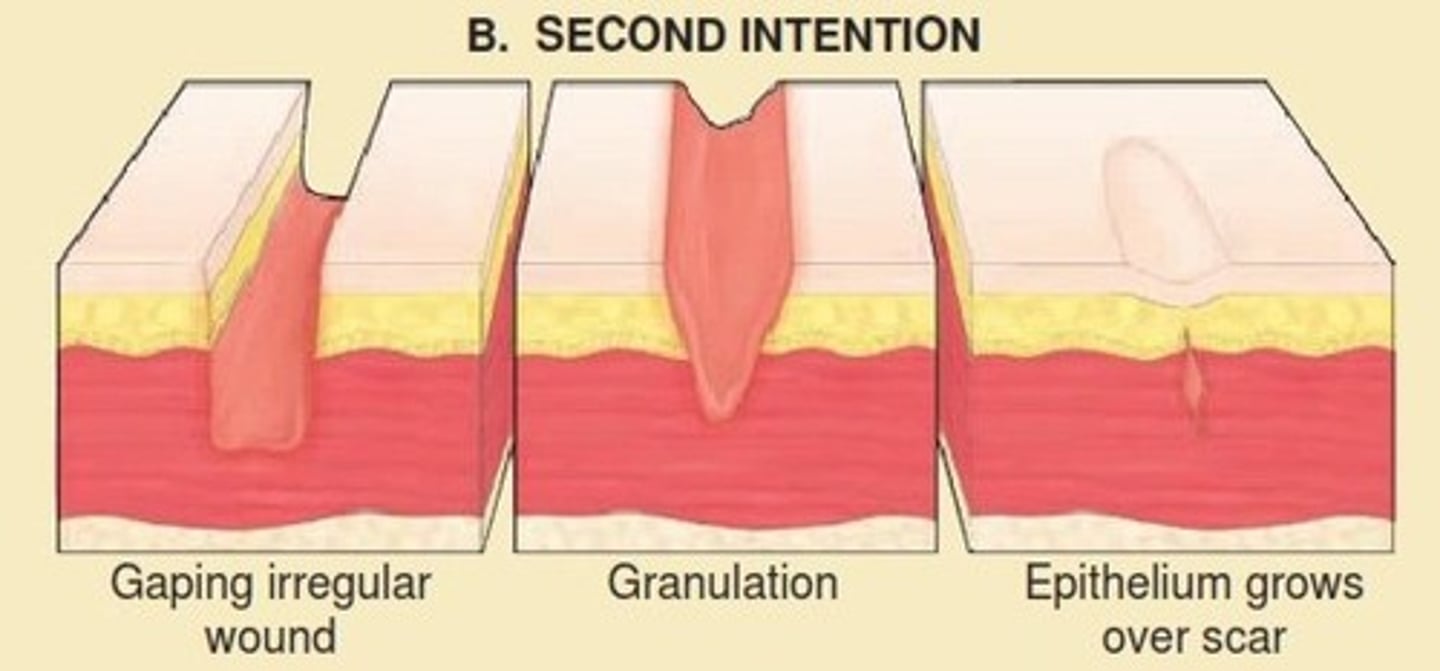
Healing by secondary intention
wound with a large defect, left open to fill in and heal naturally. It is the same process of healing as with primary intention but there is increased scarring and granulation tissue.
When does healing and repair take place with respect to inflammation?
At the same time.
Virchow's triad
- describes the three broad categories of factors that are thought to contribute to thrombosis.
1. Damaged endothelium
2. Hyper coagulation
3. Altered blood flow.