uc davis ; chem placement
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
scientific notation rules
- if the number is positive, the decimal moves to the right.
e.g.: 6.66 x 10^-6 = 0.00000666
- if the number is negative, the decimal moves to the left.
e.g.: 6.66 x 10^6 = 6660000
unit conversion calculations
- metric system acronym: KHDUDCM ;
> kilo (10^3)
> hecto (10^2)
> deca (10)
> unit (1)
> deci (10^-1)
> centi (10^-2)
> milli (10^-3)
density calculations
- density = mass/volume ; d = m/v
- when doing stoich, remember to use the correct units
percent mass calculations
mass percent tells you the percentage of each element that makes up the compound.
- mass % = (mass of chemical/total mass of compound) x 100
> in grams
solving for mass when not given masses ;
- mass % = (molar mass of element/total molecular mass of compound) x 100
> in (g/mol)
> find molar masses in periodic table!
reading and balancing chemical formulas
- subscripts of formulas may not be changed; only allowed to change coefficients ;
1. write out number of atoms below each side of equation
e.g.: __C4H10 + __O2 → __CO2 + __H2O
C = 4 C = 1
H = 10 H = 2
O = 2 O = 3
2. then, toy with the coefficients until the numbers balance on each side.*
e.g.: 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
C = 8 C = 8
H = 20 H = 20
O = 26 O = 26
*you can simplify if all coefficients have a common divisor that produces whole numbers.
types of reactions
- synthesis: two or more chemicals combine to form a more complex product.
> A + B → AB
- decomposition: a compound is broken down into smaller chemical species
> AB → A + B
- single replacement: one element is displaced from a compound by another element
> A + BC → AC + B
- double replacement: two compounds exchange bonds or ions in order to form different compounds
> AB + CD → AD + CB
- combustion: type of redox reaction in which a combustible material reacts with an oxidizer to form oxidized products and generate heat. one example is the burning of naphthalene:
> C10H8 + 12 O2 → 10 CO2 + 4 H2O + e
stoichiometry
the relation between the quantities of substances that take part in a reaction or form a compound (typically a ratio of whole integers)
- mole → mole conversions
> ratios of moles to be used in the equation can be found in a balanced chemical reaction, if provided.
e.g.: 2 NaClO(s) → 2 NaCl(s) + O2(g); (0.0253 mol NaClO/1)(2 mol NaCl/2 mol NaClO) = 0.0253 mol NaCl
- gram ←→ mole conversions
> use molar mass to convert between grams and moles
e.g.: (2.59 g CaCO3/1)(1 mol CaCO3/100.1g CaCO3) = 0.026 mol CaCO3; 100.1 = molar mass of CaCo3
- avogadro's number: the # of particles found in 1 mole of substance = 6.0221 x 10^23 particles per mole
molarity calculations
- molarity = moles of solute/liters of solution ; M = mol/L
- solute = substance dissolved
- solvent = liquid substance is dissolved in
ideal gas law
- (pressure in atm)(volume of gas in L) = (moles)(ideal gas constant)(temperature in K) ; pv = nrt
> 1 L = 1 m^3
- r = 0.0821 (L x atm)/(mol x K)
- combined gas law: p1v1/t1 = p2v2/t2
> derive laws from this, such as charles's and boyle's laws
- 1 mole of gas at STP occupies a volume of 22.4 L
atomic/molecular structures and properties
- atoms consist of protons and neutrons in the nuclei and shell(s) of electrons surrounding it
> electrons in outer shells have more energy than electrons closer to nucleus
- covalent bonding: two atoms share a pair of valence shell electrons to complete octet
> bonds are polar or nonpolar
- ionic bonding: bond between atoms of differing electronegativity; atom gives up valence electron(s) to another atom; forms ions
> stronger than covalent bonds (within crystal networks) b/c involves complete transfer of electrons
- hydrogen bonding: intermolecular bond between hydrogen atom and one that is extremely electronegative (h = fon)
- metallic bonding: valence electrons are delocalized over many atoms, producing an electronic band structure
- intermolecular bonds: bonds between different particles
- intramolecular bonds: bonds within the particle itself
- valence electrons: an outer shell electron that can participate in the formation of a chemical bond if the outer shell is not closed
- dipole: separation of positive and negative charges
> shared electrons will spend more time closer to atoms that have a greater electronegativity
> the greater the dipole movement, the greater the separation of charges
- VSEPR theory: the valence electron pairs surrounding an atom tend to repel each other and will adopt an arrangement that minimizes this repulsion, determining the molecular geometry of the particle
- molecular geometry:
> linear: 2 atoms bonded to central atom; 180°
> trigonal planar: 3 atoms bonded to central atom; 120°
> tetrahedral: 4 atoms bonded to central atom; 109.5°
> trigonal bipyramidal: 3 atoms bonded to central atom, 1 lone pair; 109.5 (107.8°)
> octahedral: 6 atoms bonded to central atom; 90°, 180°
mass number, protons, neutrons, and electrons
- mass number: total number of protons and neutrons in an atomic nucleus
- protons: found in nucleus, positive charge
> determine element
- neutrons: found in nucleus, neutral charge
> purpose is to allow protons to bond instead of repelling each other
> changing # of neutrons creates isotopes
- electrons: found in orbitals, negative charge
> changing # of electrons changes charge of atom, creating cations and anions
> electrons orbit in pairs and spin in opposite directions
> s subshell = 1 orbital (2e)
> p subshell = 3 orbitals (6e)
> d subshell = 5 orbitals (10e)
> f subshell = 7 orbitals (14 e)
> full configuration: 1s2-2s2-2p6-3s2-3p6-4s2-3d10-4p6-5s2-4d10-5p6-6s2-4f14-5d10-6p6-7s2
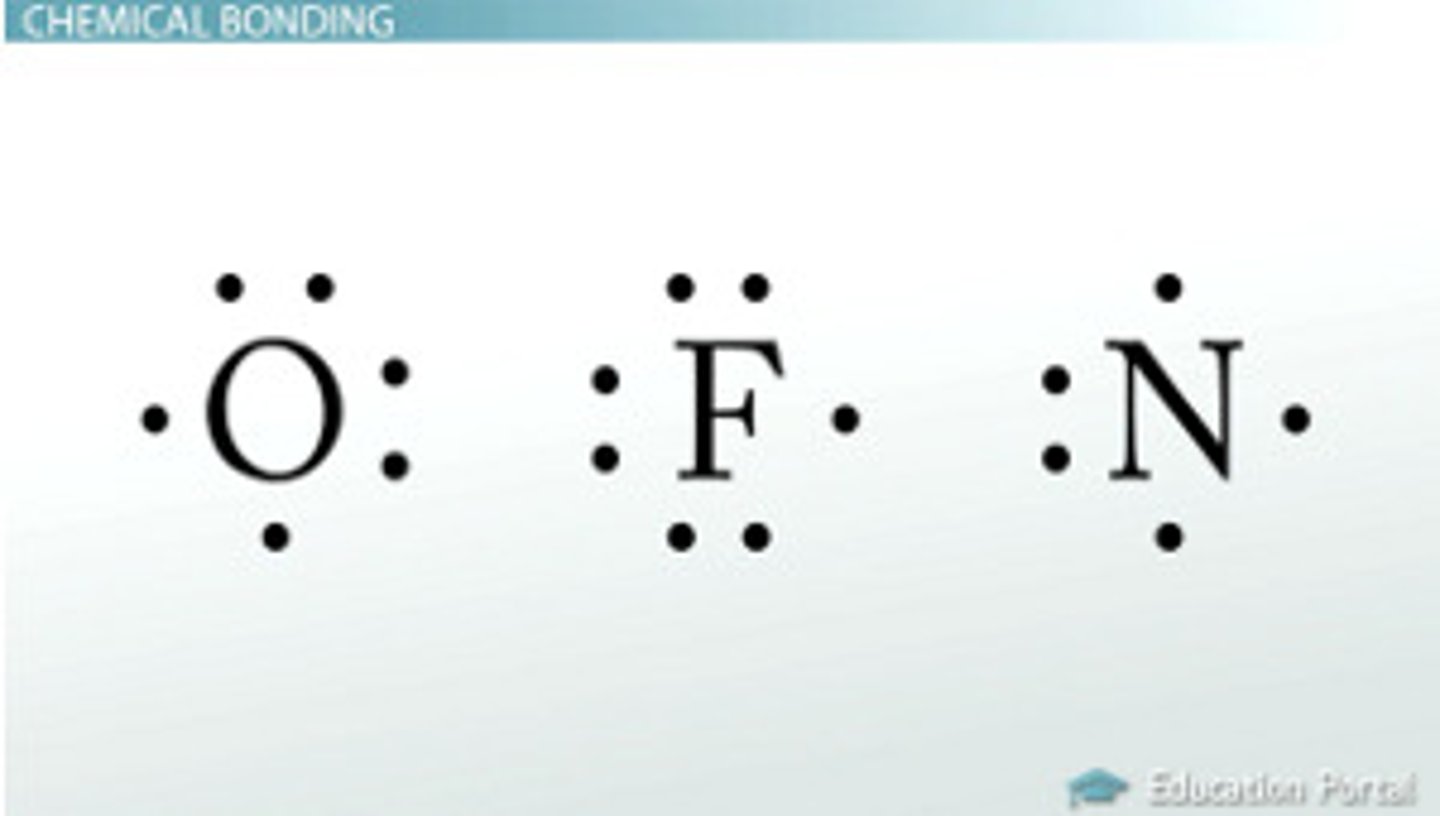
lewis electron dot structures
- a drawing that represents chemical bonds between atoms as shared or transferred electrons; the valence electrons of atoms are represented as dots
- dots represent unbonded electrons (lone pairs)
- dashes represent bonds (- is single; = is double, etc.)
- number of valence atoms of each atom dictates how many connections need to be formed; oxygen has 6 valence electrons, so it needs to have 6 connections, either through bonds or lone pairs.
> different from the octet requirement! number of connections doesn't have to equal 8, but the number of electrons assigned must be! (1 bond = 2 e-; 2 bonds = 4 e-, etc.)
- if each atom's octet requirements are not met, double and triple bonds must be drawn
- if molecule is an ion, # of e- will be taken/added to structure as needed
- the least electronegative atom is made the center of the structure (if possible and if it makes sense; e.g. would NOT for HCl or H2O)
polar bonds
- a type of covalent bond between atoms that differ in electronegativity. the shared electrons are pulled closer to the more electronegative atom, making one slightly negative and the other slightly positive.
- the electronegativity difference between the two atoms is from 0.4 - 1.6.
- polar molecules known as "dipoles"
- polar bonds can exist in nonpolar molecules; e.g. CO2. the polar bonds are arranged in a symmetric form, so that the "pulls" on each side cancel each other out.
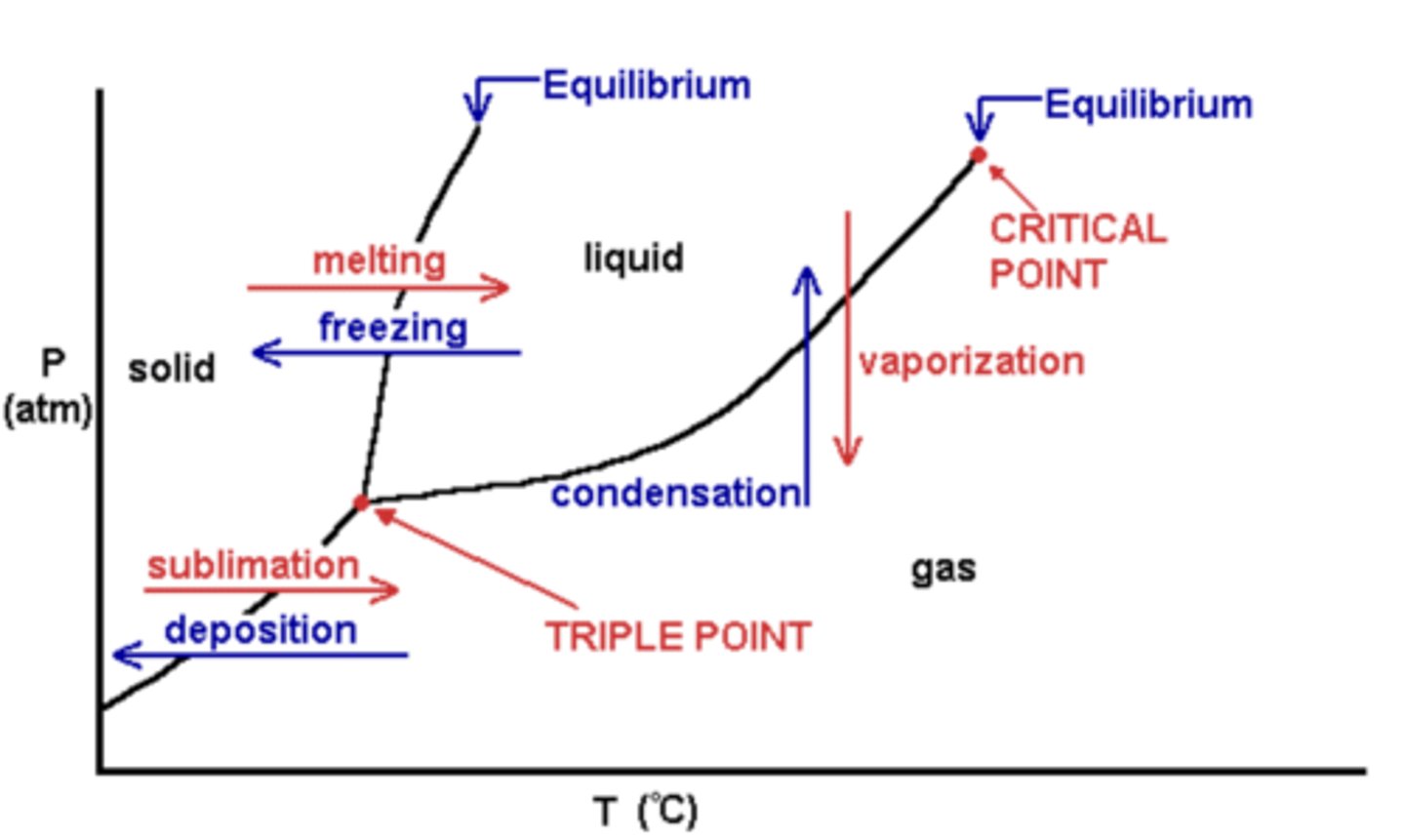
phase diagrams
- a graph of pressure versus temperature that shows which phase a substance exists in under different conditions of temperature and pressure
- a triple point will exist where all 3 line segments intersect; this is the only combination of temperatures and pressures that allow all three phases to exist simultaneously
- critical temperature: the temperature above which a substance cannot exist as a liquid
- critical pressure: the pressure needed to liquefy a gas at its critical temperature
- critical point: the combination of critical temperature and critical pressure
states of matter
- solid, liquid, and gas
> plasma is an atom stripped of all electrons (gaseous)
- when the states of matter of the same substance are held at different temperatures, the attractive force strength between its molecules is the same for all states
- when the states of matter of the same substance is held at the same temperature, the average kinetic energy between all the states is the same
> however, solids and liquids have a higher total amount of kinetic energy because they have more molecules to assign the average kinetic energy to
- not all particles contain the same amount of kinetic energy (see maxwell-boltzmann distribution)
- important properties of liquids ;
> surface tension: resistance of a liquid to create new surfaces as the result of an imbalance of attractive forces (more attracted to itself than its surroundings)
> viscosity: resistance of a liquid to flow (how "thick" it is; more viscous, the thicker). directly related to strength of IMFs
> capillary action: attraction of surface of liquid to surface of solid (related to surface tension); liquid will rise in a tube if attraction is strong enough
- important properties of solids ;
> electrical conductivity: in order for a substance to be conductive, it must have charged particles (e.g. ions) and be able to move around (unlike most solids). the only conductive solids are metallics b/c of e- sea
> solubility: "like dissolves like" (general rule only tho)
periodicity and group trends of elements
- metals ;
> metallic luster, can conduct heat and electricity, are malleable and ductile, not soluble in natural form
> metal oxides usually form bases, some can react to both acids and bases
> alloys ;
> mixture of two or more metals
> lower melting point than those of its components
> harder than metals that compose it
- metalloids (semi-metals) ;
> intermediate properties btwn metals and non-metals
> semiconductors
- non-metals ;
> high ionization energy and electronegativity
> poor conductors of heat and electricity
> brittle and no metallic luster
> lower melting and boiling points
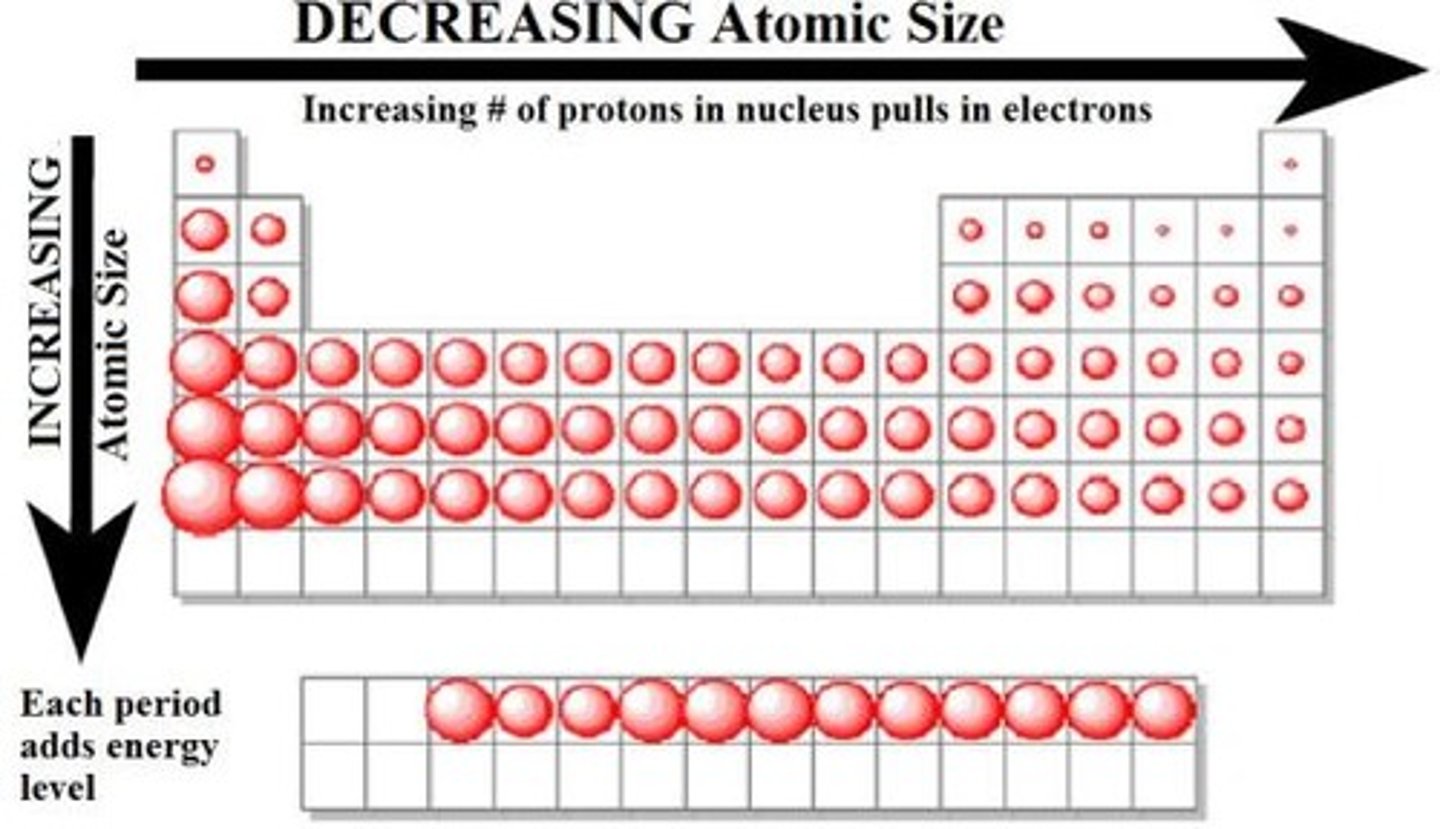
- periodic table general trends ;
> acid-forming properties increase from left to right
> atomic radii increase from left to right
> atomic radii increase from top to bottom in a group
> first ionization energy increases left to right
> metallic properties are greatest on left and decrease to the right
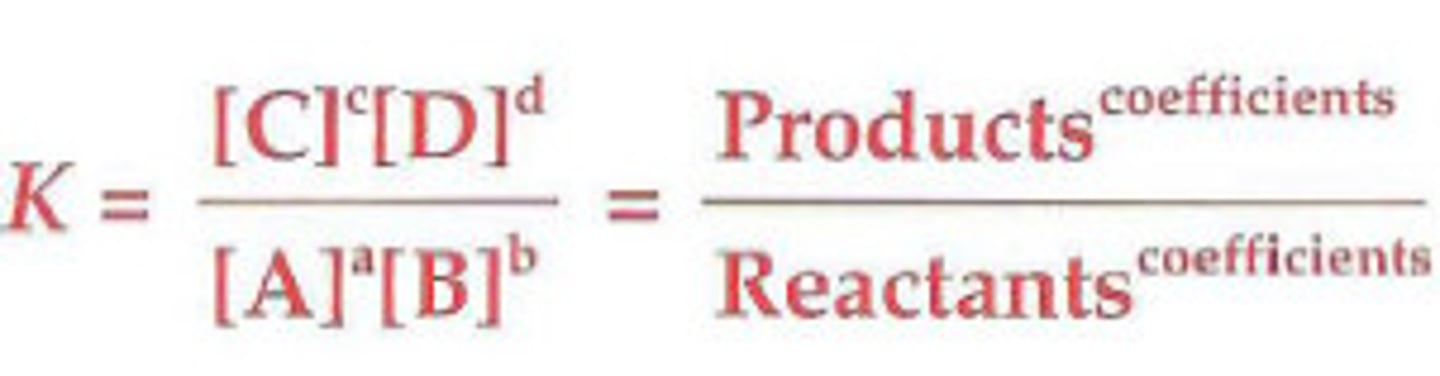
equilibrium expression
- the expression giving the ratio between the products and reactants. it is equal to the concentration of each product raised to its coefficient in a balanced chemical equation and multiplied together, divided by the concentration of the product of reactants to the power of their coefficients
- forward reaction will be placed over backwards reaction
- concentration of reactants/products directly affects reaction rate
- keq (k1/k2) is the equilibrium constant
> if the keq is large, equilibrium will not be reached until the concentration of original reactants are small and those of products large
> small keq means equilibrium occurs almost at once and relatively little product is produced
le chatelier's principle
- when a stress is applied to a system in dynamic equilibrium, the system changes in a way that relieves the stress
- effect of changing concentration ;
> all concentrations will change until new equilibrium point is reached with same value of keq
> if concentration in forward action is increased, equilibrium is displaced to right, favoring forward rxn
- effect of temperature ;
> if temp is increased, equilibrium is shifted in direction that absorbs heat
> as temp changes, keq will change with it
- effect of pressure ;
> affects gasses only
> as # of molecules inc., pressure increases
> as pressure is increased, reaction will favor the direction that decreases the pressure
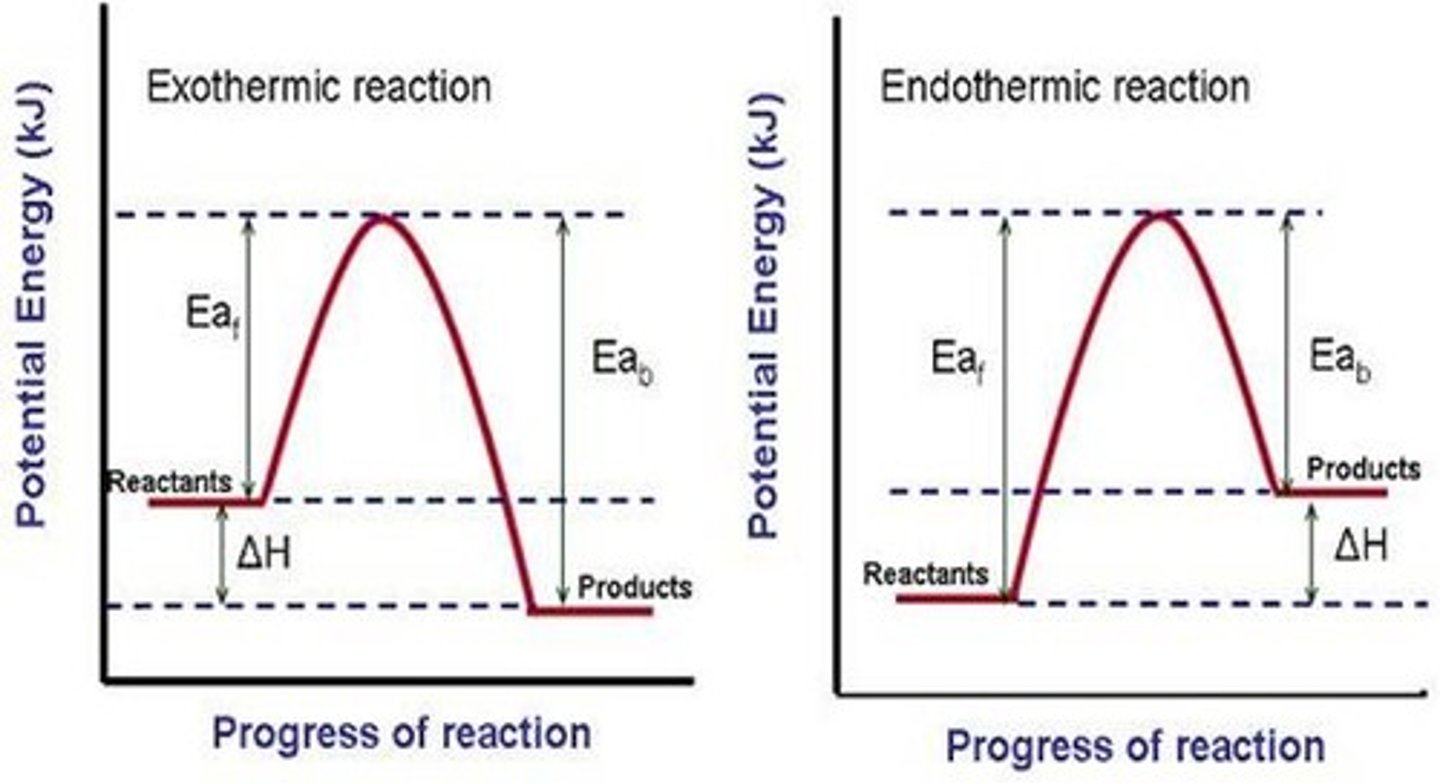
potential energy diagram
- a diagram that shows the changes in potential energy that takes place during a chemical reaction
- exothermic reaction, energy is lost (products energy levels lower than reactants); opposite for endothermic
- catalyst will decrease activation energy ("hump")
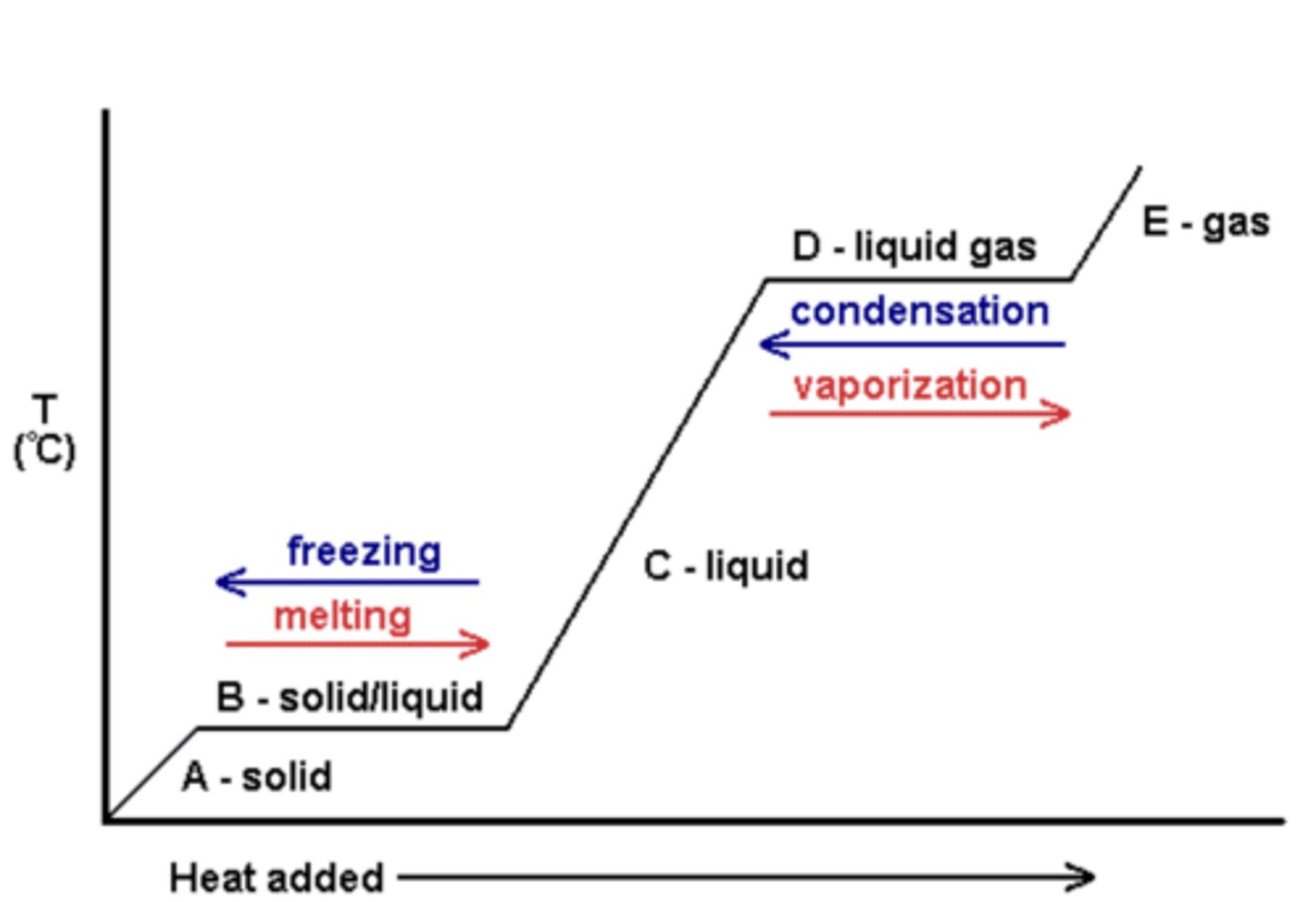
heating curve
- a plot of temperature versus time for a substance where energy is added at a constant rate
- for the "sloped parts," use q=mcat equation; if energy is being lost, -q=mcat
- for the "flat parts," convert mass of substance to moles, and using stoich, find the amount of energy used to phase change by using the provided heat of fusion/enthalpy
- net energy will be negative if releasing energy
kinetic molecular theory
- matter in all forms is composed of extremely small particles
- the particles of matter are in constant motion
- when these particles collide with each other or with the walls of the container, there is not loss of energy
pH concepts
- pH can be defined as -log[H+], where [H+] is the concentration of hydrogen ions expressed in mol/L
> 100 = 10^2, so base 10 = 2, which gives that acid a pH level of 2
> this means that the acidity of acids goes up by a factor of ten each "level" (the closer to 0, ofc)
- pOH is the negative log of the OH- concentration; pOH = -log[OH-]
- pH scale ranges generally from 0 - 14; 7 is neutral, 0-6 is acidic, and 8-12 is basic (superacids and superbases exceed the normal scale)
- sum of pH and pOH must always equal 14