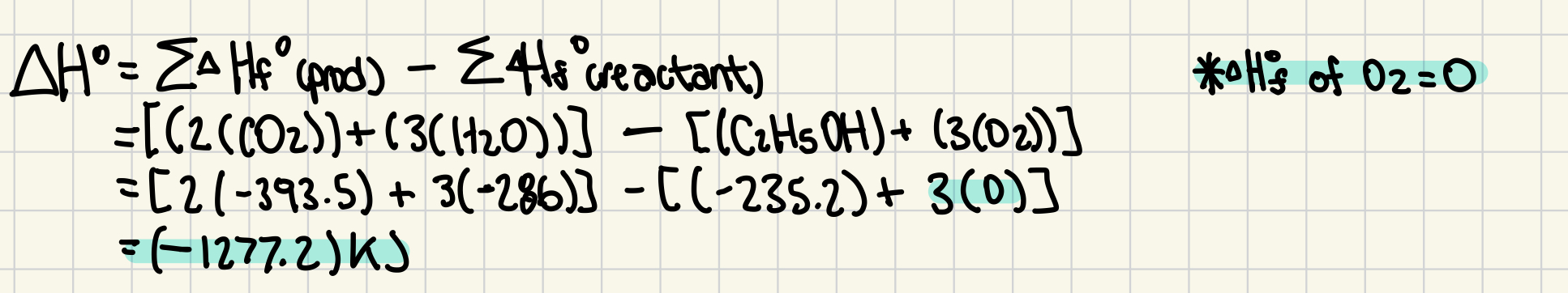SCH4U U3 Thermochemistry & Kinetics
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
87 Terms
1. What is heat?
a. | A measure of the average kinetic energy of entities in a substance. |
b. | The total amount of kinetic and potential energy in a substance. |
c. | The transfer of thermal energy from a warm object to a cooler object. |
d. | None of the above. |
c. | The transfer of thermal energy from a warm object to a cooler object. |
2. A chemical system in which neither energy nor matter can flow into or out of a system is described as
a. | a closed system. | c. | an isolated system. |
b. | an open system. | d. | a chemical system. |
c. | an isolated system. |
3. The change in energy of a system (ΔEsys) is often described by the term q (heat), but this assumes that
i. the temperature of the system is 0oC.
ii. the work done in the system is zero.
iii. the molar enthalpy of the system does not change.
iv. the volume of the system does not change.
a. | i. only | d. | both i. and ii. |
b. | ii. only | e. | both ii. and iv. |
c. | iii. only | ||
d. | both i. and ii. |
i. the temperature of the system is 0oC.
ii. the work done in the system is zero.
4. The specific heat capacity of a substance is
a. | the quantity of heat required to raise the temperature of one mole of a substance by 1o Celsius or Kelvin. |
b. | the quantity of heat required to raise the temperature of one gram of a substance by 1o Celsius or Kelvin. |
c. | the quantity of heat required to raise the temperature of one molecule of a substance by 1o C or K. |
d. | the quantity of heat required to raise the temperature of a substance by 1o Celsius or Kelvin. |
b. | the quantity of heat required to raise the temperature of one gram of a substance by 1o Celsius or Kelvin. |
5. To conduct an experiment, a scientist places a small piece of aluminum in a test tube and adds sodium hydroxide solution. Bubbles of gas are produced and the outside of the test tube becomes hot to the touch. Which of the following is true?
a. | The reaction is endothermic, and the system gains energy from the surroundings. |
b. | The reaction is exothermic, and the system loses energy to the surroundings. |
c. | The reaction is endothermic, and the system loses energy to the surroundings. |
d. | The reaction is exothermic, and the system gains energy from the surroundings. |
b. | The reaction is exothermic, and the system loses energy to the surroundings. |
6. A chemical reaction is carried out in a coffee cup calorimeter. The temperature of the water changes from 25.2 °C to 19.8 °C. Which statement is correct?
a. | The water loses energy, so the reaction is exothermic. |
b. | The water gains energy, so the reaction is exothermic. |
c. | The water loses energy, so the reaction is endothermic. |
d. | The water gains energy, so the reaction is endothermic. |
c. | The water loses energy, so the reaction is endothermic. |
7. An enthalpy change is
a. | the difference in the kinetic energy of the reactants and the products in a chemical change. |
b. | the difference in the potential energy of the reactants and the products in a chemical change. |
c. | the difference in enthalpies of the reactants and the products in a chemical change. |
d. | the sum of the PE and KE of the products. |
e. | the sum of the PE and KE of the reactants. |
a. | the difference in the kinetic energy of the reactants and the products in a chemical change. |
8. Four common materials used to make pots for cooking are copper, stainless steel, aluminum, and glass. Pots made out of which material will heat up the fastest?
ccopper = 0.385 J/(g °C), cstainless steel = 0.510 J/(g °C), caluminum = 0.900 J/(g °C), cglass = 0.753 J/(g °C)
a. | aluminum | c. | stainless steel |
b. | glass | d. | copper |
d. | copper |
9. What quantity of heat is required to raise the temperature of 0.500 g of graphite by 25.0 °C?
(cgraphite= 0.710 J/(g °C))
a.8.88 J b. 0.355 J c. 70.4 J d. 35.5 J |
a.8.88 J
Q = mcΔT
Q = (0.5)(0.710)(25)
Q = 8.875
Q = 8.88J
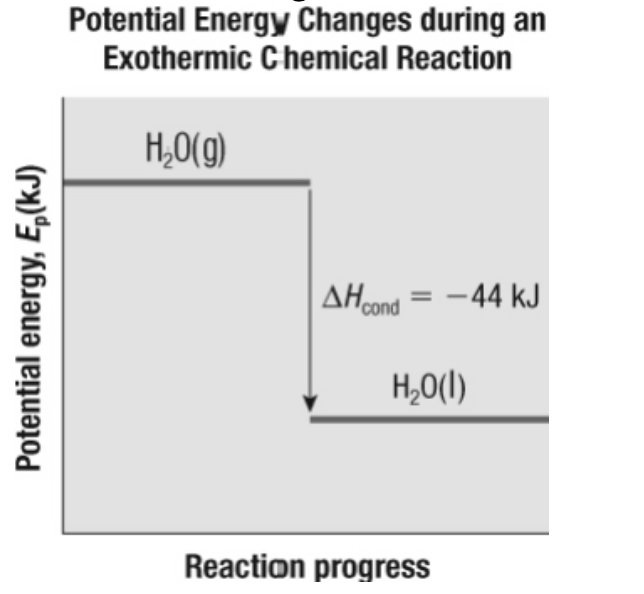
10. Which equation correctly summarizes the information in this figure?
a. | H2O(g) → H2O(l) + 44 kJ |
b. | ΔHcond = 44 kJ |
c. | H2O(g) → H2O(l), ΔH = 44 kJ |
d. | all of the above |
a. | H2O(g) → H2O(l) + 44 kJ |
11. Given the equation:
N2(g) + 2 O2(g) → 2 NO2(g), ΔH = 68 kJ
Which of the following is correct?
a. | N2(g) + O2(g) → NO2(g), ΔH= –68 kJ |
b. | N2(g) + O2(g) → NO2(g), ΔH = 68 kJ |
c. | NO2(g) → N2(g) + O2(g), ΔH = –34 kJ |
d. | NO2(g) → N2(g) + O2(g), ΔH = 34 kJ |
c. | NO2(g) → N2(g) + O2(g), ΔH = –34 kJ |
12. For an endothermic reaction, which of the following are true?
i. Hproducts > Hreactants
ii. Hreactants > Hproducts
iii. ΔH is -ve
iv. ΔH is +ve
a. | i. and iii. | c. | ii. and iii. |
b. | i. and iv. | d. | ii. and iv. |
b. | i. and iv. |
13. Which of the following reactions is exothermic?
a. | H2(g) + 1/2 O2(g) → H2O(g) ΔH = –243 kJ/mol |
b. | 1/2 N2(g) + 1/2 O2(g) + 90.7 kJ → NO(g) |
c. | CO(g) + 111 kJ→ C(s) + 1/2 O2(g) |
d. | 1/2 H2(g) + 1/2 I2(g) → HI(g) ΔH = +26 kJ/mol |
e. | H2O(g) →H2(g) + 1/2 O2(g) – ΔH = –243 kJ/mol |
a. | H2(g) + 1/2 O2(g) → H2O(g) ΔH = –243 kJ/mol |
14. What property would be appropriate to measure the reaction rate in the following reaction:
a. | Δ in conductivity | d. | change in pressure |
b. | change in mass | e. | change in temperature |
c. | change in colour | ||
d. | change in pressure |
15. The initial rate of the a reaction
a. | is dependent on the initial concentrations of the reactants. |
b. | is always less than the average rate of the reaction. |
c. | can be determined by studying the balanced equation. |
d. | is equal to the instantaneous rate after 1.0 min. |
a. | is dependent on the initial concentrations of the reactants. |
16. The slope of a tangent on an graph of concentration vs. time will give which quantity?
a. | instantaneous rate | d. | chemical rate |
b. | average rate | e. | all of the above |
c. | initial rate | ||
a. | instantaneous rate |
17. If you compare the concentrations of reactant A at two different time intervals, the type of rate that can be determined is the:
a. | instantaneous rate | d. | chemical rate |
b. | average rate | e. | all of the above |
c. | initial rate | ||
b. | average rate |
18. In order for a collision to be effective, which of the following statements are true?
a. | The collision geometry of the reactants must be favourable |
b. | The collision must occur with sufficient energy |
c. | The collision must occur at the correct time |
d. | Both a) and b) are correct |
e. | All of a), b), and c) are correct |
d. | Both a) and b) are correct |
19. Which of the following is NOT a factor that affects the rate of a reaction?
a. | pressure of gases | d. | temperature |
b. | [ ] of a solution | e. | all of the above |
c. | nature of reactants | ||
e. | all of the above |
20. Increasing the surface area increases the rate of a reaction because
a. | the number of collisions increases. |
b. | the activation energy decreases. |
c. | the fraction of collisions that are effective increases. |
d. | Only a) and b) are true. |
e. | All a), b), and c) are true |
a. | the number of collisions increases. |
21. A catalyst increases the reaction rate by
a. | providing an alternative, low-energy pathway for the reaction |
b. | increasing the kinetic energy of the reactant molecules |
c. | increasing the number of collisions between reactant molecules |
d. | colliding with the reactant molecules, increasing their energy, and adjusting their orientation |
a. | providing an alternative, low-energy pathway for the reaction |
22. Increasing the temperature of the reactants increases
a. | the number of collisions. |
b. | the rate of the reaction. |
c. | the fraction of collisions that are effective. |
d. | Only a) and b) are true |
e. | All a), b), and c) are true |
e. | All a), b), and c) are true |
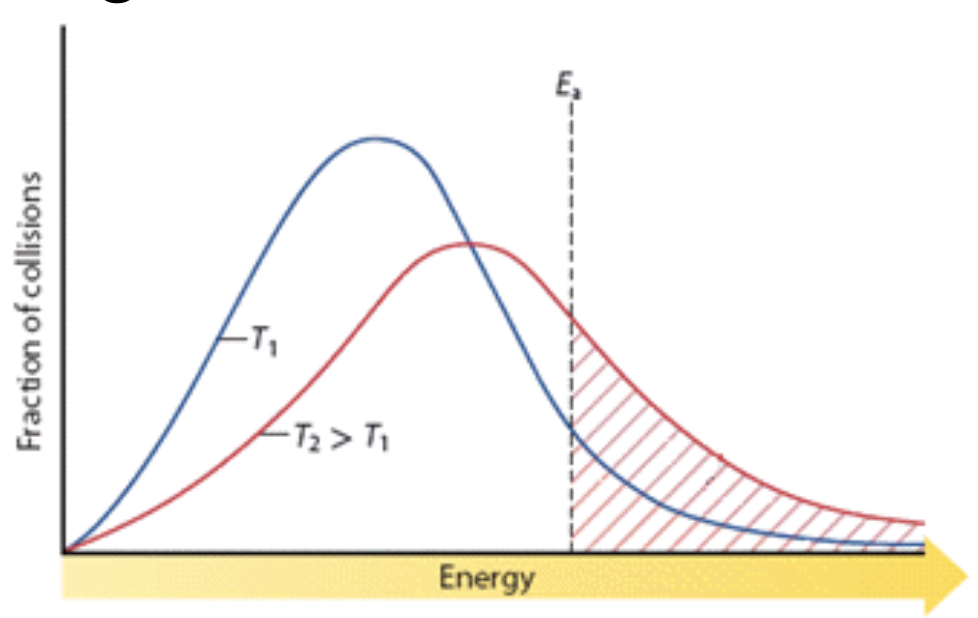
23. Consider the following activation energy diagram.
From the activation energy diagram it is seen that the reaction rate will ___i___ at ___ii___ temperature. The statement above is completed by information in row:
Row | ___i___ | ___ii___ |
A. | decrease | lower |
B. | increase | higher |
C. | decrease | higher |
D. | increase | lower |
a. A b. B c. C d. D |
b. B
B. | increase | higher |
At higher temperature T2, the red curve shows a greater area under the curve beyond Ea (to the right of the dashed line). This shaded area represents the fraction of particles that have enough energy to react.
At lower temperature T1 the area beyond Ea is smaller, meaning fewer particles can overcome the activation energy barrier.
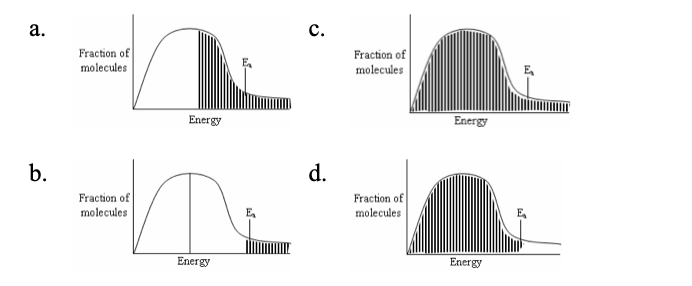
24. Maxwell Boltzmann diagrams give the relationship between fraction of molecules and energy. Which of the following graphs correctly represents an effective collision if the fraction of molecules capable of effective collision is given by the shaded position?
a. | c. |
b. | d. |
b.
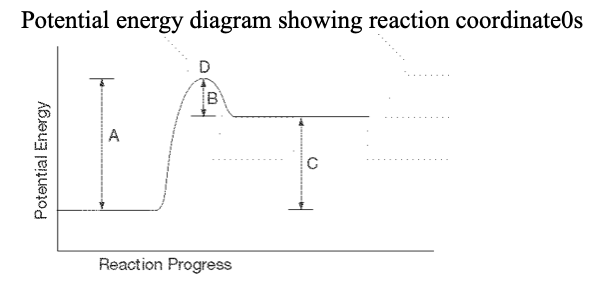
25. For the above PE diagram, which letter represents the enthalpy change of the reaction?
a. A b. B c. C d. D |
c. C
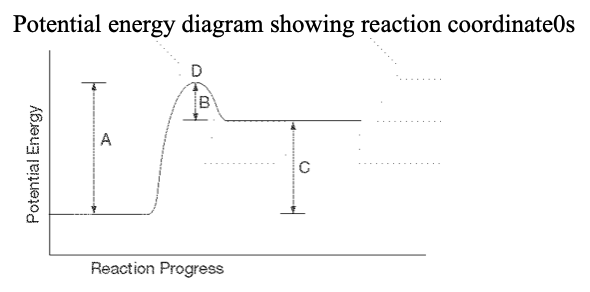
26. For the above PE diagram, ...which letter represents the activation energy of the forward reaction?
a. A b. B c. C d. D |
a. A
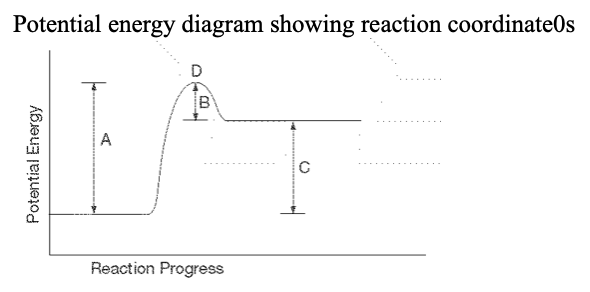
27. ... which letter represents the location of the activated complex?
a. A b. B c. C d. D |
d. D
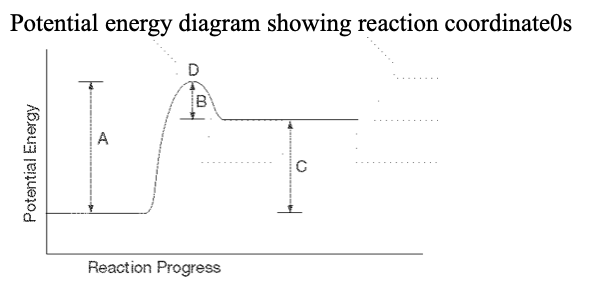
28. ... what kind of reaction is represented?
a. | nuclear | c. | exothermic |
b. | endothermic | d. | barimetric |
b. | endothermic |
29. The activated complex
a. | is an unstable molecule. |
b. | has the maximum potential energy possible. |
c. | may continue on to produce products. |
d. | may revert to reactants. |
e. | All of the above. |
e. | All of the above. |
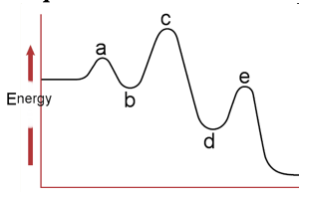
30. Which letter represents the activated complex of the rate determining step?
a. A
b. B
c. C
d. D
c. C
31. Given the following reaction: N2(g) + 3 H2(g) →2 NH3(g)
If the concentration of hydrogen decreases by 3 mol/L in 45 seconds, what is the corresponding rate of disappearance of nitrogen?
a. | 0.022 mol/L*s | d. | 0.16 mol/L*s |
b. | 0.067 mol/L*s | e. | 0.26 mol/L*s |
c. | 0.080 mol/L*s | ||
a. | 0.022 mol/Lïs |
For every 1 mol of N2\ that reacts, 3 mol of H2 react.
Given:
Hydrogen decreases by 3 mol/L in 45 seconds.
Rate of disappearance of H2 = 3 mol/L ÷ 45 s = 0.0667 mol/L·s
From the balanced equation:
N2(g) + 3 H2(g) → 2 NH3(g)
This means for every 1 mol of N2 used, 3 mol of H2 are used.
So,
Rate of disappearance of N2 = Rate of disappearance of H2 ÷ 3
Rate of disappearance of N2 = 0.0667 mol/L·s ÷ 3 = 0.0222 mol/L·s
Since H₂ disappears 3 times faster than N₂ (because of the 3:1 ratio), the rate of N₂ disappearance is 1/3 the rate of H₂.
32. Consider the chemical reaction:
H2SO4(aq) +2 NaOH(aq) → 2 H2O(l) + Na2SO4(aq)
If the sulphuric acid is reacting at a rate of 1.60 mol/L*s, the sodium sulphate will be produced at a rate of
a. | 0.40 mol/Lïs | d. | 2.40 mol/Lïs |
b. | 0.80 mol/Lïs | e. | 3.20 mol/Lïs |
c. | 1.60 mol/Lïs | ||
c. | 1.60 mol/Lïs |
33. A reaction is found to be second order with respect to reactant A. What would be the effect on the rate if the concentration of A were doubled?
a. | The rate would double |
b. | The rate would quadruple |
c. | The rate would decrease by half |
d. | The rate would increase in an unpredictable way |
e. | The concentration of A has no effect on the rate |
b. | The rate would quadruple |
34. An initial rate of reaction is usually obtained by measuring
a. | the rate at which products are consumed. |
b. | the rate at which reactants are produced. |
c. | the rate at which reactants are consumed. |
d. | the temperature of the solution. |
e. | the mass and volume of the products. |
c. | the rate at which reactants are consumed. |
35. What is the average rate of production of carbon dioxide for the system between 2.0 and 4.0 minutes, CH4(g) = 2O2(g) → CO2 + 2H2O(g) , if the concentration of carbon dioxide is 2.5 mol/L after 2.0 minutes and 7.2 mol/L after 4.0 minutes?
a. | 0.426 mol/(L·min) | d. | 2.35 mol/(L·min) |
b. | 1.18 mol/(L·min) | e. | 42.6 mol/(L·min) |
c. | 0.952 mol/(L·min) | ||
d. | 2.35 mol/(L·min) |
Given:
Reaction:
CH₄(g) + 2 O₂(g) → CO₂(g) + 2 H₂O(g)
CO₂ concentration at 2.0 minutes = 2.5 mol/L
CO₂ concentration at 4.0 minutes = 7.2 mol/L
Step 1: Find the change in concentration of CO₂
Change in [CO₂] = 7.2 mol/L – 2.5 mol/L = 4.7 mol/L
Step 2: Find the change in time
Change in time = 4.0 min – 2.0 min = 2.0 min
Step 3: Calculate average rate of production
Average rate = Change in [CO₂] ÷ Change in time
Average rate = 4.7 mol/L ÷ 2.0 min = 2.35 mol/L·min
Final Answer:
Average rate of production of CO₂ = 2.35 mol/L·min
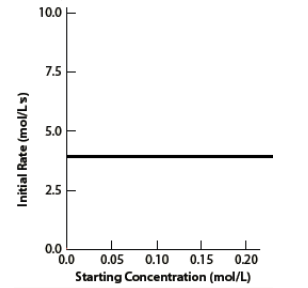
36. Given the following graph of rate vs. concentration,
What must be the exponent of this reactant in the rate law for this process?
a.0 b. 1 c. 2 d. ½ e. 3 |
a.0
This graph shows a horizontal line for initial rate as the concentration increases. That means:
The rate stays constant, even though the concentration changes.
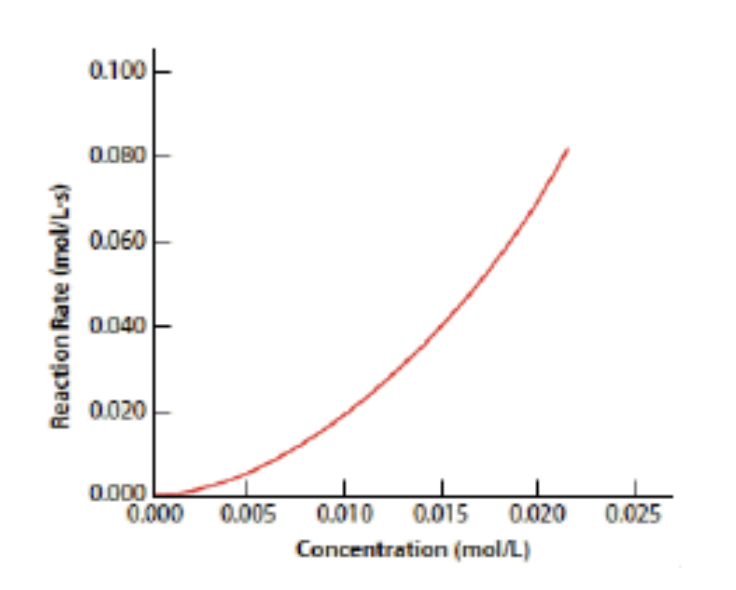
37. Experiments were carried out to determine the effect of changing concentration on the initial rate of reaction for a given reaction. Given the data in the graph, what is the order of reaction with respect to this reactant?
a.0 b. 1 c. 2 d. 3 |
c. 2
second-order, the graph would be a curve that steepens, showing a quadratic relationship.
38. Given the equation 2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g), which of the following could be used to monitor the rate of the reaction?
a. | The decrease in mass of sodium. |
b. | The volume of hydrogen gas produced. |
c. | The pH of the solution. |
d. | All of the above. |
d. | All of the above. |
39. The rate constant for a reaction is
a. | the sum of the exponents in the rate law equation. |
b. | temperature dependent. |
c. | determined from the balanced equation. |
d. | the exponent used to describe the relationship between the initial concentration of a reactant and the reaction rate. |
b. | temperature dependent. |
40. If the rate law for a reaction is determined to be rate = k[X]1[Y]2[Z]0, then the overall order of the reaction is
a.0 b. 1 c. 2 d. 3 |
d. 3
sum of the exponents
41. A plausible reaction mechanism (one that might work) must satisfy the following requirements:
a. | The balanced equation must be predicted by summing the elementary steps, and the mechanism must agree with the theoretically predicted equation. |
b. | The balanced equation must be produced by summing the elementary steps, and the mechanism must agree with the experimentally determined rate law. |
c. | The balanced equation must be produced by summing the elementary steps, and the mechanism must agree with the theoretically determined rate law. |
d. | The balanced equation must be produced by summing the reaction intermediates, and the mechanism must agree with the experimentally determined rate law. |
b. | The balanced equation must be produced by summing the elementary steps, and the mechanism must agree with the experimentally determined rate law. |
42. The number of particles that would usually "collide" in an elementary step are
a. 1-2 b. 3-4 c. 5-6 |
d. Unlimited e. no particles need to collide |
a. 1-2
43. A theoretical reaction is given: A + B2 + C AB + CB
The proposed mechanism for this reaction is as follows.
A + B2 → AB + B (slow)
B + C → BC (fast)
Based on this mechanism, the rate law expression for this reaction is
a. | rate = k[A][B2] | c. | rate = k[B2][C] |
b. | rate = k[A][B2][C] | d. | rate = k[A][C] |
a. | rate = k[A][B2] |
44. The radioisotope Pb-212 has a rate constant of 0.0654h-1. What is the half-life for this isotope?
a. | 15.3 h | d. | 10.6h |
b. | 0.0654 h-1 | e. | 0.050 h-1 |
c. | 12120 h-1 | ||
d. | 10.6h |
To find the half-life, use the formula:
t₁/₂ = ln 2 ÷ k (calculus: (1/2) × N₀ = N₀ × e^(–kt) otherwise just remember 0.693/k)
Given:
k = 0.0654 h⁻¹
So,
t₁/₂ = 0.693 ÷ 0.0654 ≈ 10.6 hours
Correct answer: d. 10.6 h
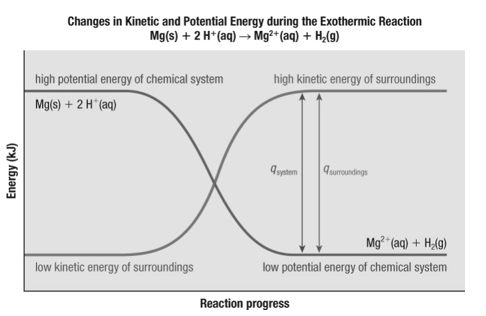
45. Which statement is correct about the graph?
Energy is lost …
a. from the surroundings into the system, indicating an exothermic reaction. |
b. from the system into the surroundings, indicating an exothermic reaction. |
c. from the system into the surroundings, indicating an endothermic reaction |
d. from the surroundings into the system, indicating an endothermic reaction. |
b. from the system into the surroundings, indicating an exothermic reaction.
46. Chlorine atoms can increase the rate of decomposition of ozone in the atmosphere. The mechanism for this reaction is:
(1) Cl + O3 → ClO + O2
(2) O3 → O + O2
(3) ClO + O → Cl + O2
(4) O + O3 → O2 + O2
If the rate law for this reaction is rate = k[O3], the ______ reaction is the rate determining step.
a. | first |
b. | second |
c. | third |
d. | fourth |
b. | second |
*Which of the following is true of the system and its surroundings ni aprocess in which ΔEsys= (+100kJ)?
a.The system loses 100kJ of energy and the surroundings gain 100kJ of energy.
b. The system and the surroundings both lose 100kJ of energy.
c. The system gains 100kJ of energy and the surroundings lose 100kJ of energy.
d. The system and the surroundings both gain 100kJ of energy.
c. The system gains 100kJ of energy and the surroundings lose 100kJ of energy.
*An exothermic reaction is one where
a. heat is transferred from the surroundings into a system
b. heat is transferred from a system into the surroundings
c. kinetic energy is transformed into potential energy
d. there is no transfer of heat
b. heat is transferred from a system into the surroundings
*Enthalpy changes depend on the temperature and presssure under which a process occurss. However, the enthalpy change will be different at different presssures. Therefore, chemists often deal with standard enthalpy of reaction ΔrHo. The temperature and pressure conditions for standard enthalpy are:
a. 0°C and 1 atm
b. 25°C and 1 atm
c. 2.5°C and 10kPa
d. 2.5 K and 100 Pa
e. 25K and 1 atm
b. 25°C and 1 atm
*Which of the following substances (with specific heat capacity provided) would show the least temperature change upon absorbing 100.0 J of heat?
a. 10.0g Ag, CAg= 0.235J/g°C
b. 10.0g H2O, CH2O=4.18 J/g°C
c. 10.0g ethanol, Cethanol=2.42J/g°C
d. 10.0g Fe, CFe= 0.449J/g°C
e. 10.0 g Au, CAu= 0.128J/g°C
b. 10.0g H2O, CH2O=4.18 J/g°C
*Calculate the amount of heat (in KJ) necesssary to raise the temperature of 47.8 g benzene by 57.0 K. The specific heat capacity of benzene is 1.05 J/g°C
a. 1.61 kJ
b. 16.6 kJ
c. 2.59 kJ
d. 2.86 kJ
e. 3.85 kJ
d. 2.86 kJ
q = m × c × ΔT
q = 47.8 g × 1.05 J/g·°C × 57.0 °C
q = 2854.59 J = 2.85 kJ
treat 57 K as 57°C
*Determine the specific heat capacity of an alloy that requires 59.3 kJ to raise the temperature of 150.0g alloy from 298 K too 398K
a. 4.38 J/g°C
b. 2.29 J/g°C
c. 3.95 J/g°C
d. 2.53 J/g°C
e. 1.87 J/g°C
c. 3.95 J/g°C
Recall 1°C =1K, they just have different starting points (0 deg C vs -273K)
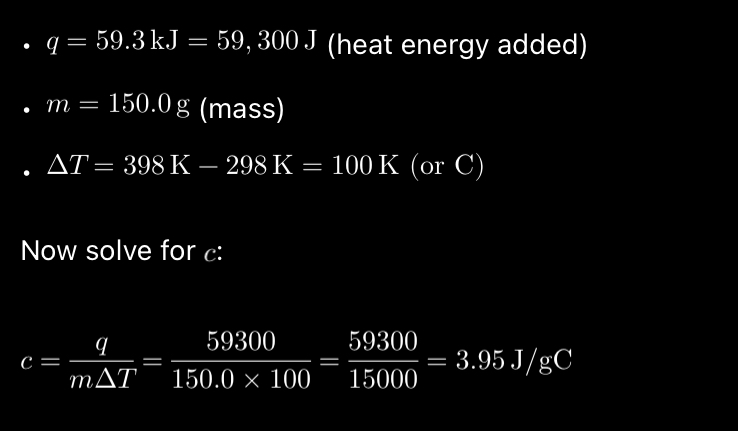
*Given this information: N2(g) + O2 → 2NO(g); ΔHrxn= +80.5kJ
if Hp denotes the enthalpy of products and Hf denotes the enthalpy of reactants, then
a. Hp > Hf
b. Hp < Hf
c. Hp = Hf
d. Hp >/= Hf
a. Hp > Hf
*When 6.000 moles of glucose are combusted, 16 815 kJ of energy is produced. Which of the following chemical equations is an equivalent representation of this process?
a. 6C₆H₁₂O₆(s) + 36O₂(g) → 36CO₂(g) + 36H₂O(ℓ) ΔH = (–2802.15 kJ)
b. 6C₆H₁₂O₆(s) + 36O₂(g) → 36CO₂(g) + 36H₂O(ℓ) ΔH = 16815 kJ
c. C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(ℓ) ΔH = (–16815 kJ)
d. C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(ℓ) ΔH = (–2802.15 kJ)
d. C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(ℓ) ΔH = (–2802.15 kJ)
Balanced chem equation for 1, and divide 16815 by6 mols so you have delta H per mole
*Which of the following statements is false concerning the heating and cooling curve of water?
a. Energy is absorbed to increase the kinetic energy of liquid water.
b. Energy is absorbed when liquid water evaporates.
c. Energy is released when liquid water freezes.
d. Energy is absorbed when H-bonds between water molecules are broken.
e. Energy is absorbed when bonds inside the water molecules are broken.
e. Energy is absorbed when bonds inside the water molecules are broken.
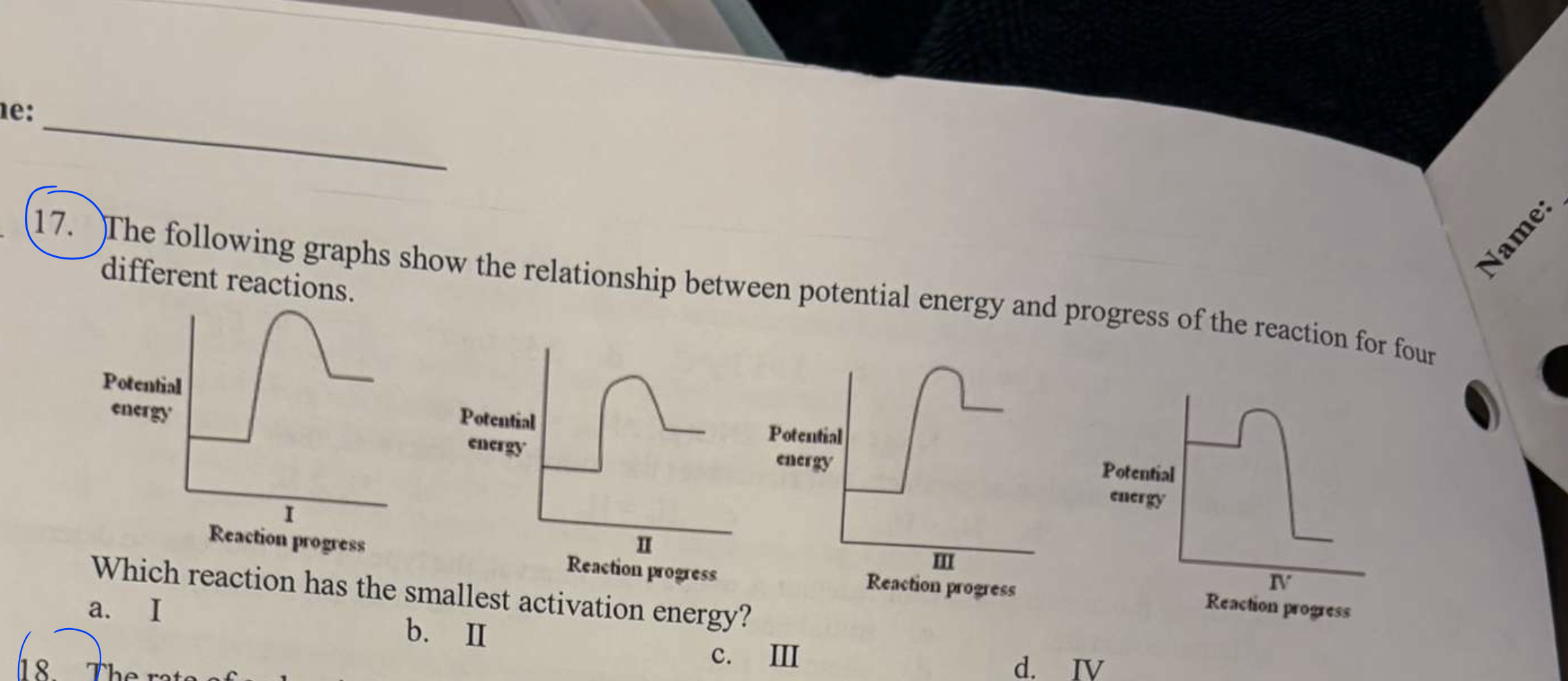
*The following graphs show the relationship between potential energy and progress of the reaction for four different reactions.
Which reaction has the smallest activation energy?
a. I b. II c. III d. IV
d. IV
*X grams of sucrose give an enthalpy change of Y kJ/mol on combustion. What is the effect on enthalpy change when the mass of the sucrose is doubled?
a. The enthalpy change remains constant.
b. The enthalpy change doubles.
c. Enthalpy change is reduced to half.
d. ΔH stays the same.
e. Enthalpy becomes zero.
b. The enthalpy change doubles.
*The enthalpy of formation of an element in its most stable state, under standard conditions, is
a. constant.
b. changing.
c. the same as the reverse process.
d. infinity.
e.zero
e.zero
*The rate of a chemical reaction is doubled for every 10°C rise in temperature. This is because of a(n)
a. decrease in activation energy.
b. increase in activation energy.
c. increase in # of ineffective collisions.
d. increase in the # of effective collisions.
e. increase in enthalpy.
d. increase in the # of effective collisions.
*If we draw a potential energy diagram for an endothermic reaction, the potential energy (PE) of reactants is
a. less than PE of products.
b. equal to the PE of the products.
c. more than PE of the products.
d. equal to Eₐ of reverse reaction.
e. equal to Eₐ of forward reaction.
a. less than PE of products.
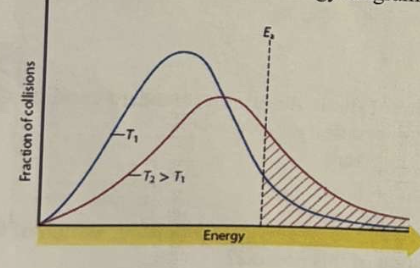
*Consider the following activation energy diagram (a Maxwell-Boltzmann distribution).
From the above activation energy diagram, it is seen that the reaction rate will ___ at ___ temperature.
A. decrease / lower
B. increase / higher
C. decrease / higher
D. increase / lower
E. stay the same / any
B. increase / higher
*A reactant is found to have an order of zero in a chemical reaction. What would be the effect on the rate if the concentration of that reactant were decreased by half?
a. The rate would double.
b. The rate would quadruple.
c. The rate would decrease by half.
d. The rate would increase in an unpredictable way.
e. The concentration has no effect on the rate.
e. The concentration has no effect on the rate.
*A reaction has the following rate law: r = k[A]²[B]
What would be the effect on the rate if the concentration of A were doubled?
a. The rate would double.
b. The rate would quadruple.
c. The rate would decrease by half.
d. The rate would increase in an unpredictable way.
e. The concentration of A has no effect on the rate.
b. The rate would quadruple.
*Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. If the rate of Cl₂ loss is 4.84 × 10⁻² M/s, what is the rate of formation of NOCl?
2 NO(g) + Cl₂(g) → 2 NOCl(g)
a. 4.84 × 10⁻² M/s
b. 2.42 × 10⁻² M/s
c. 1.45 × 10⁻¹ M/s
d. 9.68 × 10⁻² M/s
e. 1.61 × 10⁻² M/s
d. 9.68 × 10⁻² M/s
For every 1 mol of Cl2, 2 NOCLare formed, so given the rate of Cl2, you’d double it (x2) to match the ratio
*For the overall reaction CHCl₃(g) + Cl₂(g) → CCl₄(g) + HCl(g) the following reaction mechanism is determined by experimentation:
CHCl₃(g) → 2Cl(g) (fast)
CHCl₃(g) + Cl(g) → CCl3(g) + HCl(g) (slow)
CCl3(g) + Cl(g) → CCl4(g) (fast)
What is the rate law for this reaction?
a. r = k[Cl₂]
b. r = k[CHCl₃][CHCl₃]
c. r = k[CHCl₃][Cl]
d. r = k[CHCl₃][CCl₃]
e. r = k[CCl₃][Cl]
c. r = k[CHCl₃][Cl]
*Which of the following can affect the activation energy of a reaction?
a. Pressure of gases.
b. Concentration of a solution.
c. The presence of a catalyst.
d. Temperature.
e. All of the above may affect the Eₐ.
c. The presence of a catalyst.
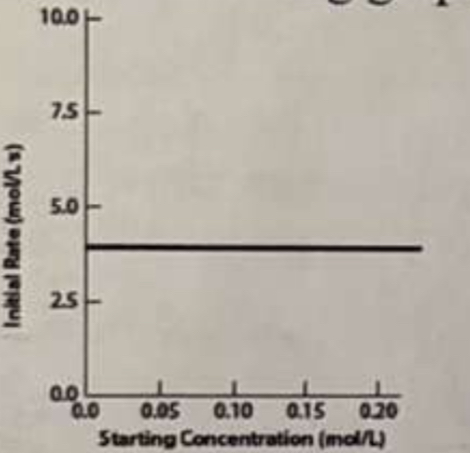
*In an experiment where the concentration of one reactant in a chemical process is varied and the rate is measured, the following graph (initial rate vs concentration) is obtained:
What must be the exponent of this reactant in the rate law for this process?
a. 1/2 b. 0 c. 1 d. 2 e. 3
b.0
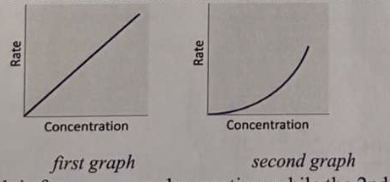
*The 1st graph is for a ___ order reaction, while the 2nd graph is for a ___ order reaction.
a. 0 ; 1
b. 1 ; 2
c. 2 ; 1
d. 1 ; 0
b. 1 ; 2
*How many half-lives are required for the concentration of reactant to decrease to 25% of its original value?
a. 1 b. 3 c. 1.5 d. 2.5 e. 2
e. 2
(After 1 half life, ur at 50, so after 2 you’ll be at 25)
*The rate constant for the first order decomposition for N2O is 3.40s-1. What is the half life of the decomposition?
a. 0.491s
b. 0.204 s
c. 0.235 s
d. 0.424 s
e. 0.294 s
b. 0.204 s

*Complete each statement
__________ is a measure of the average kinetic energy of the particles in a substance.
The energy values reported on food are Calories. A Cal is a (#) ________ calories
Temperature
1000
Match the term with the correct definition below:
a. | thermochemistry | f. | open system |
b. | ΔHof | g. | temperature |
c. | specific heat capacity | h. | isolated system |
d. | 1st law of thermodynamics | i. | Hess’s law |
e. | calorie | j. | state function |
1. property that depends only on the condition of the system at the moment, not how it got there.
2. the amount of energy needed to increase the temperature of one gram of a substance by one degree Celsius
3. a system that can exchange both matter and energy with the surroundings
4. a system that cannot exchange either energy or matter with the surroundings
5. energy can be converted from one form to another but cannot be created or destroyed
6. a measure of the average kinetic energy of all the particles of a sample of matter
7. the change in enthalpy when one mole of a compound is formed directly from its elements in their most stable state at standard ambient temperature and pressure
8. the enthalpy change of a physical or chemical process depends only on the initial and final conditions of the process
9. quantity of heat needed to raise the temperature of 1g of pure water by 1oC.
10. the study of the energy changes involved in chemical and physical processes
J state function
C specific heat capacity
F open system
H isolated system
D 1st law of thermodynamics
G temperature
B ΔHof
I Hess’s law
E calorie
A thermochemistry
Match the term with the correct definition below:
Classify each of the following reactions as exothermic or endothermic. Answer choices may be used more than once.
a. Exothermic
b. Endothermic
11. NaOH solution at 22 °C was mixed with HCl solution at 24 °C. The final solution had a temperature of 29 °C.
12. A piece of Mg was added to a test tube of acid. After reacting for 1 min the outside of the test tube was warm to the touch.
13. Ammonium nitrate was added to a beaker of water. Drops of water on the outside of the beaker solidified into ice.
14. Ba(OH)2 crystals were mixed with NH4Cl crystals at 25°C. A thermometer registers 10 °C after the reaction.
A Exothermic
A Exothermic
B Endothermic
B Endothermic
1. The combustion of propane (C3H8) requires five oxygen molecules and produces three carbon dioxide and four waters, producing (-2202) kJ/mol. Show three different ways of communicating this. (3 marks).
rxn coordinate diagrams
enthalpy diagrams
thermochemical equations
ΔH included within the equation or after it
Thermochemical equations
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g) ΔH = –2202 kJ (negative ΔH means exothermic)
ΔH can also be written as part of the products:
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g) + 2202 kJ
(This shows that heat is released — exothermic reaction)
Enthalpy Diagram
labeled diagram showing:
Y-axis: Enthalpy (H)
X-axis: Reaction progress
Reactants at a higher energy level than products
Downward arrow labeled ΔH = –2202 kJ
Shows energy is released
3. Reaction Coordinate Diagram
A diagram including:
Y-axis: Enthalpy (H)
X-axis:
Reaction coordinate Curve rising to a peak (activation energy), then falling to the products Label:
Reactants: C₃H₈ + 5O₂
Products: 3CO₂ + 4H₂O
Peak: Transition state
Drop between reactants and products: ΔH = –2202 kJ
2. A kilogram of water and a kilogram of sand are at the same temperature. Do the two substances contain the same amount of heat energy? Explain. (2 marks)
No, they do not contain the same amount of heat energy.
Water has a high specific heat capacity (4.18 J/g°C), one of the highest among common substances. This means it can store much more heat energy per gram per degree than most materials, including sand. So even if water and sand have the same mass and temperature, the water holds more heat energy due to its greater ability to store thermal energy. (If were given the C of sand use that in ur answer too and say the factit’s lower means it holds less heat energy)
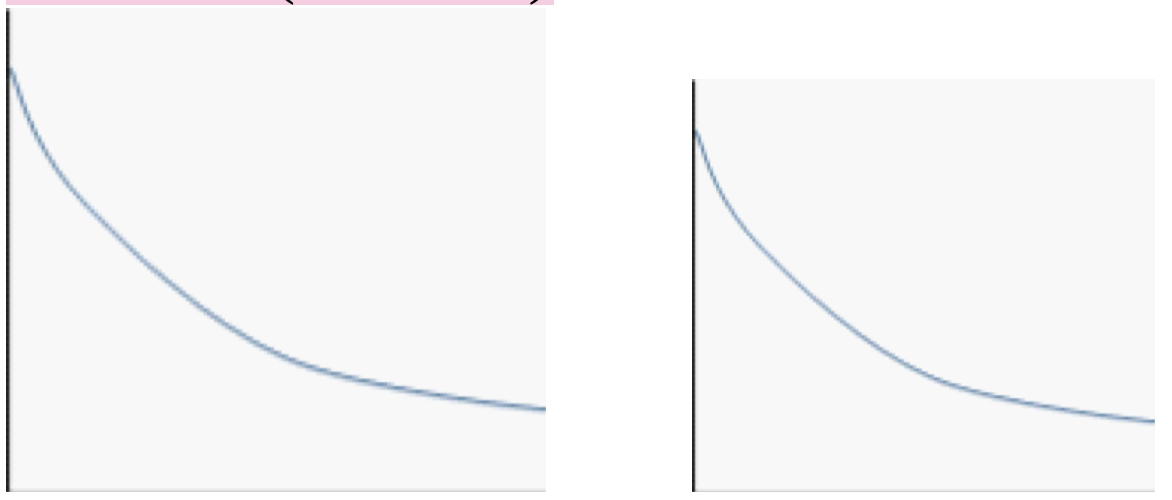
3. Use the typical rate of reaction graph (Concentration of Reactant vs. Time) and indicate (draw and explain) how to determine:
a) an average rate of reaction; and
b) an instantaneous rate of reaction (3 marks)
a) Average Rate of Reaction
How to determine:
Pick two points on the concentration vs. time graph (for example, at time t₁ and t₂).
Note their corresponding concentrations: [A]₁ and [A]₂.
Draw a straight line (secant) connecting these two points.
Formula:
Average rate = ([A]₂ - [A]₁) / (t₂ - t₁)
This gives the slope of the secant line, which represents the average rate of reaction over that time period.
b) Instantaneous Rate of Reaction
How to determine:
Choose a single point on the curve at a specific time (e.g., t = 10 s).
Draw a tangent line at that exact point.
Find the slope of the tangent line.
Formula:
Instantaneous rate = slope of the tangent line at that time
This represents how fast the reaction is happening at one specific moment.
![<p><strong>a) Average Rate of Reaction</strong></p><p class=""><strong>How to determine:</strong></p><ul><li><p class="">Pick two points on the concentration vs. time graph (for example, at time t₁ and t₂).</p></li><li><p class="">Note their corresponding concentrations: [A]₁ and [A]₂.</p></li><li><p class="">Draw a straight line (secant) connecting these two points.</p></li></ul><p class=""><strong>Formula:</strong><br>Average rate = ([A]₂ - [A]₁) / (t₂ - t₁)</p><p class="">This gives the slope of the secant line, which represents the average rate of reaction over that time period.</p><p class=""></p><p class=""><strong>b) Instantaneous Rate of Reaction</strong></p><p class=""><strong>How to determine:</strong></p><ul><li><p class="">Choose a single point on the curve at a specific time (e.g., t = 10 s).</p></li><li><p class="">Draw a tangent line at that exact point.</p></li><li><p class="">Find the slope of the tangent line.</p></li></ul><p class=""><strong>Formula:</strong><br>Instantaneous rate = slope of the tangent line at that time</p><p class="">This represents how fast the reaction is happening at one specific moment.</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/dd1b5145-cb5e-4b36-a9a5-0d3a61e08873.jpg)
4. 5.00 g of sodium hydroxide is added to enough water to reach 150 mL volume and then dissolved. Using a coffee-cup calorimeter, the temperature change of the water is measured to be 2.2oC.
a) Calculate the heat released per gram of sodium hydroxide dissolved. (3 marks)
b) bonus: how does the wording ‘5.00 g of sodium hydroxide is added 150 mL of water’ effect the answer? (1 mark)
a)
Given:
Mass of NaOH = 5.00 g
Volume of water = 150 mL
Temperature change (ΔT) = 2.2°C
Specific heat capacity of water = 4.18 J/g·°C
Assume the density of water = 1.00 g/mL → so 150 mL = 150 g of water
Step 1: Calculate total heat released (q):
q = m × c × ΔT
q = 150 g × 4.18 J/g·°C × 2.2°C
q = 1379.4 J
Step 2: Calculate heat released per gram of NaOH:
1379.4 J ÷ 5.00 g = 275.88 J/g
Final Answer: 276 J/g
b) The wording “5.00 g of sodium hydroxide is added to 150 mL of water” implies that the NaOH is added to 150 g of water, making the total mass of the solution around 155 g. However, if the calculation only uses the mass of water (150 g), it slightly underestimates the total heat absorbed, because the added NaOH also contributes mass and may slightly affect the heat capacity of the solution.
5. A sample of H2O is heated, and its temperature rises from -5oC to 110oC.
a) Draw and label a sketch graph for this process and indicate on the graph the state of the sample and the process occurring (what is happening) at each segment of the graph. (4 marks)
b) Calculate the energy expended in boiling 15g of water into steam.(2 marks)
c) Calculate the energy expended in heating 15g of water from 5oC to 20oC. (2 marks)
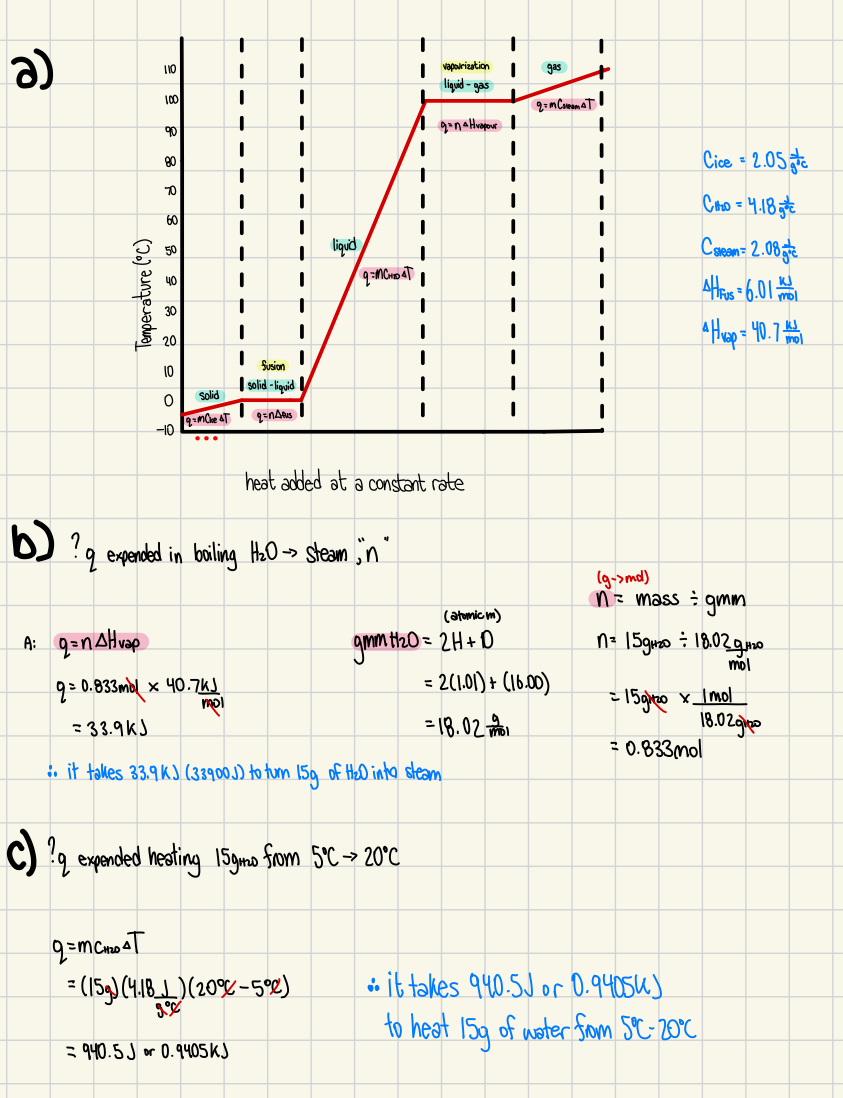
*a) Calculate the energy expended in melting 25g of ice into water (2 marks)
b) Calculate the energy expended in heating 25g of water at 95oC to steam at 110oC

6. Differentiate between the factors leading to an effective collision and an ineffective collision. (3 marks +)
Effective collisions occur when reactant particles collide with sufficient energy (at least the activation energy) and the correct orientation, allowing bonds to break and new ones to form, resulting in a chemical reaction.
Ineffective collisions happen when particles lack enough energy or collide at the wrong angle, so no reaction occurs.
Factors that increase effective collisions include higher temperature, greater concentration, and larger surface area, while the opposite conditions lead to more ineffective collisions and a lower reaction rate.
Catalysts also increase the number of effective collisions by lowering the activation energy, so more particles have enough energy to react, even though the total number of collisions stays the same.
7. Determine the enthalpy (ΔH) of the following reaction:
2 H2S(g) + SO2(g) → 3 S(s) + 2 H2O(g)
given the following equations: (5 marks)
H2O(g) → H2(g) + ½ O2(g); ΔH = 241.8 kJ
H2S(g) → H2(g) + S(s); ΔH = 20.6 kJ
S(s) + O2(g) → SO2(g); ΔH = (–296.8) kJ

8. Calculate the enthalpy change for the reaction given: (4 marks)
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
Standard enthalpies of formation ( Hf°) are:
C3H8(g) = (–103.85) kJ/mol;
CO2(g) = (–393.5) kJ/mol;
H2O(g) = (–285.8) kJ/mol
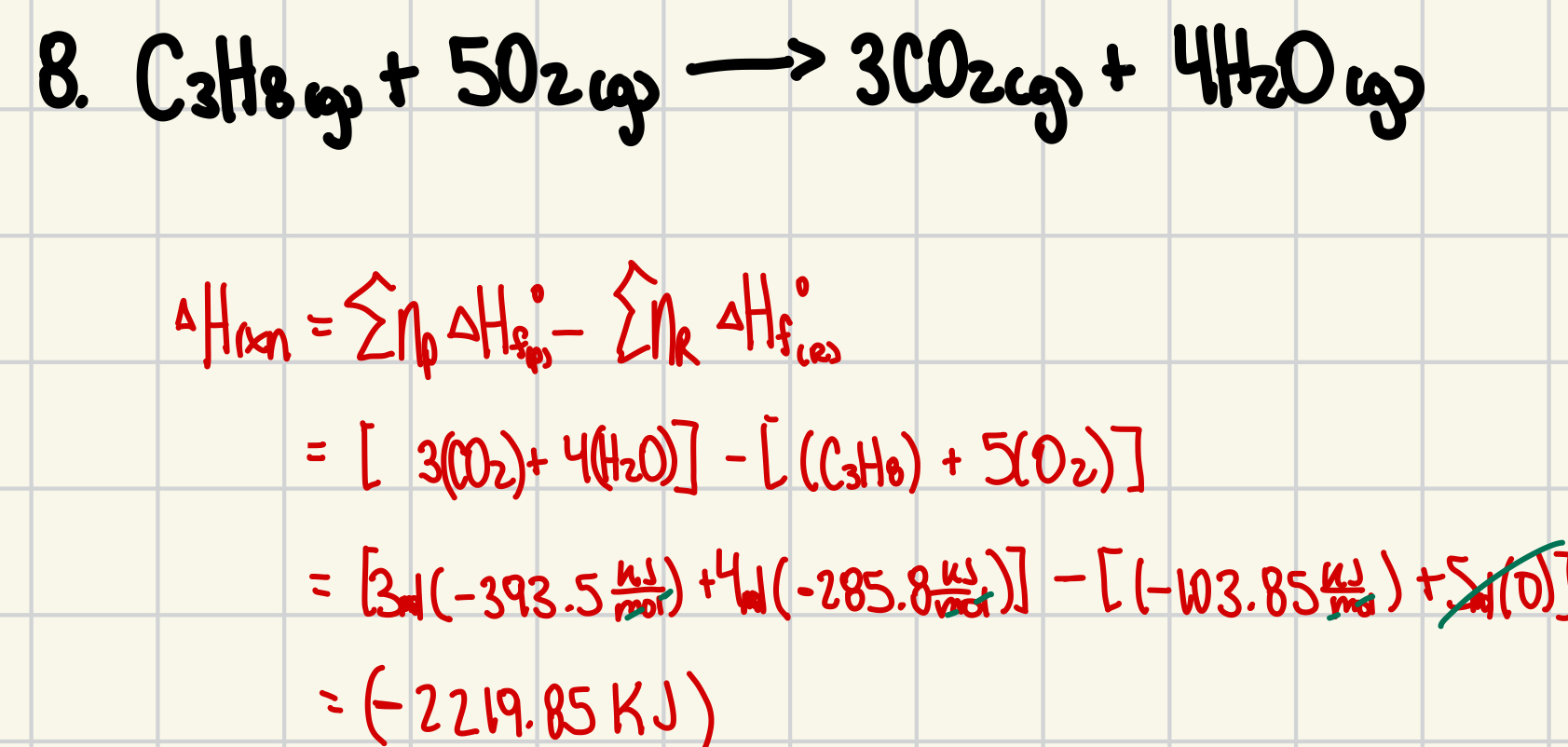
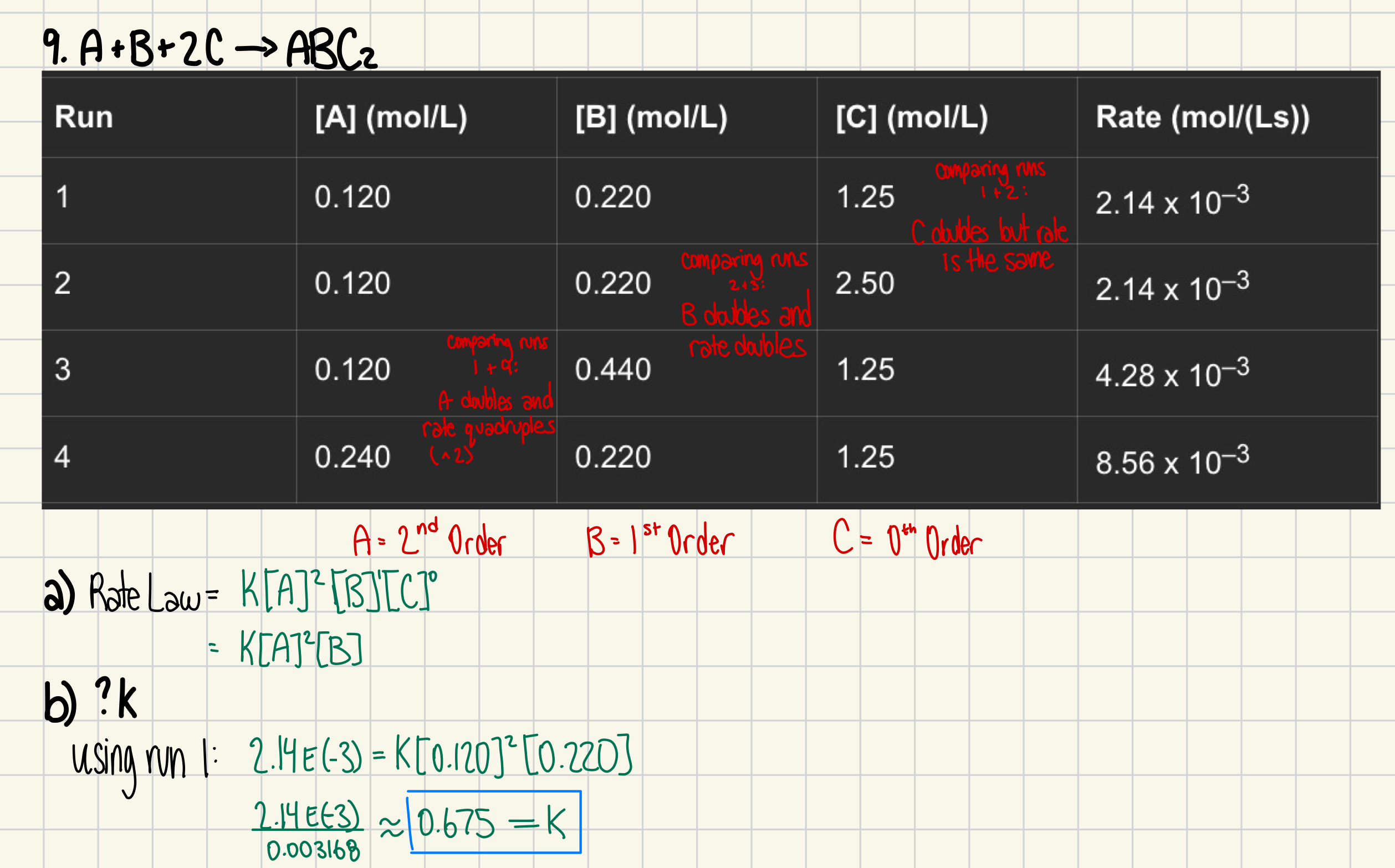
9. For the reaction A + B + 2 C → ABC2, the following data were obtained:
Run | [A] (mol/L) | [B] (mol/L) | [C] (mol/L) | Rate (mol/(Ls)) |
1 | 0.120 | 0.220 | 1.25 | 2.14 x 10–3 |
2 | 0.120 | 0.220 | 2.50 | 2.14 x 10–3 |
3 | 0.120 | 0.440 | 1.25 | 4.28 x 10–3 |
4 | 0.240 | 0.220 | 1.25 | 8.56 x 10–3 |
a) Determine the rate law for this reaction (explain your reasoning). (3 marks)
b) Determine the value of the rate constant. (2 marks)
*Use the following data to calculate the rate law for the system, and a value for the rate constant. (5 marks)
NO(g) + H₂(g) → HNO₂(g)
Experiment | NO (mol/L) | H₂ (mol/L) | Initial Rate of Reaction (mol/L·s) |
|---|---|---|---|
1 | 0.001 | 0.004 | 0.002 |
2 | 0.002 | 0.004 | 0.008 |
3 | 0.003 | 0.004 | 0.018 |
4 | 0.004 | 0.001 | 0.008 |
5 | 0.004 | 0.002 | 0.016 |
6 | 0.004 | 0.003 | 0.024 |
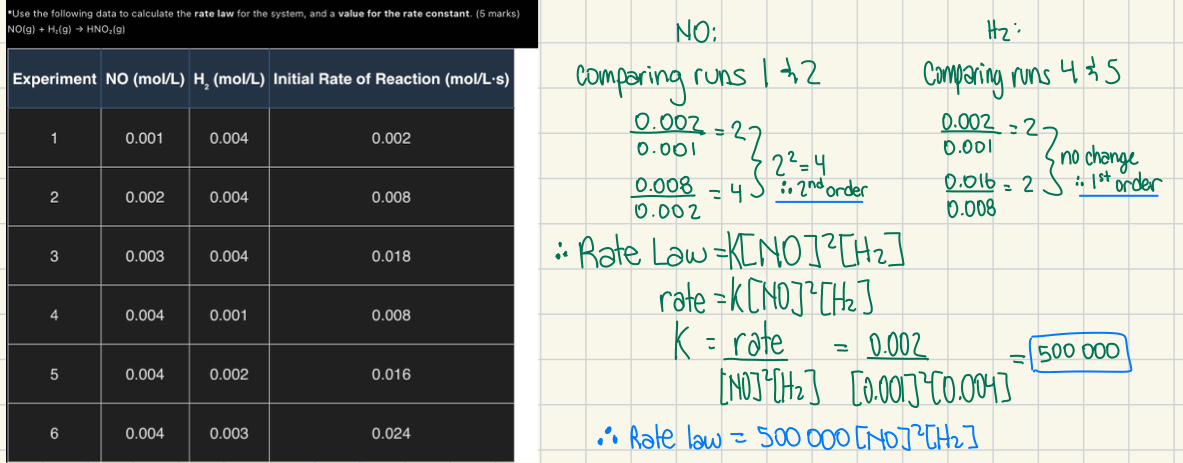
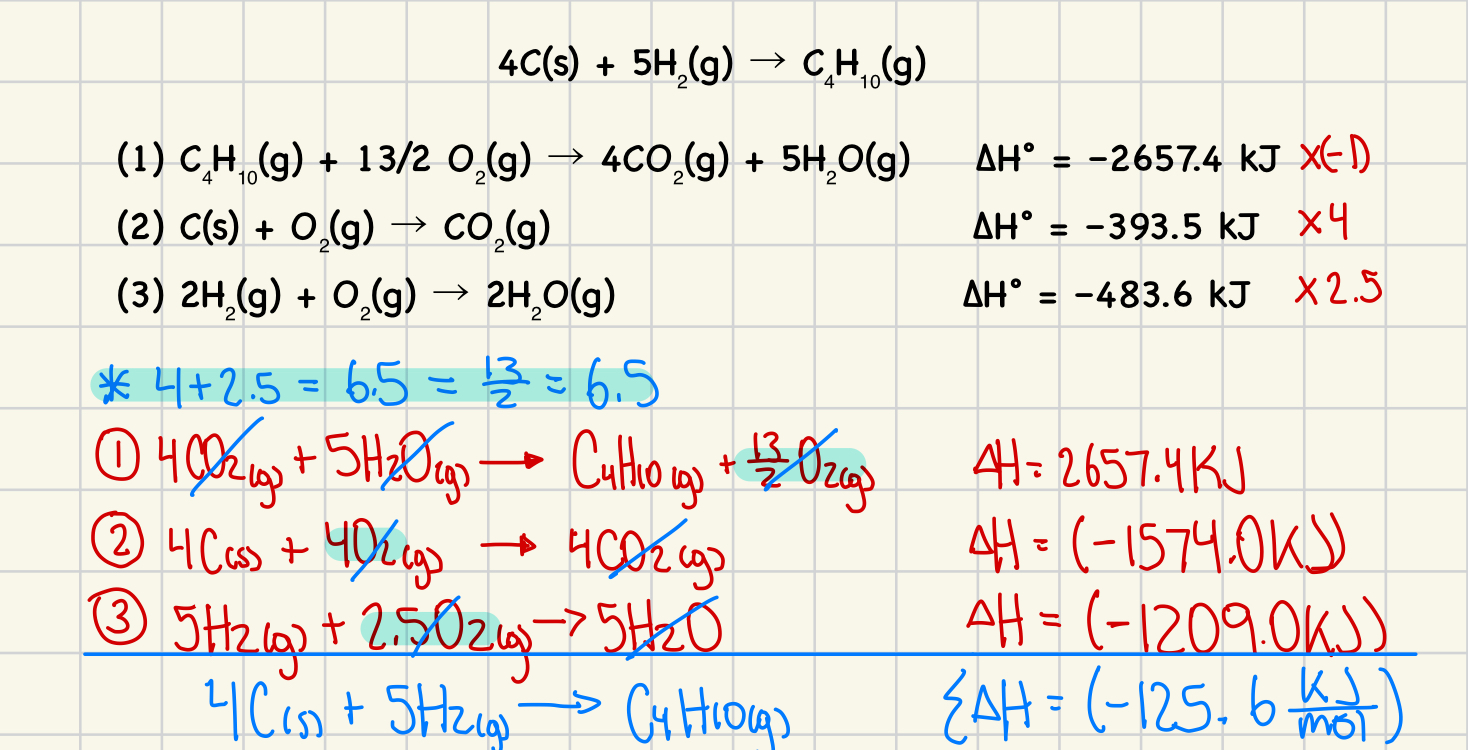
*What is the enthalpy change for the formation of one mole of butane gas (C₄H₁₀) from its elements?
4C(s) + 5H₂(g) → C₄H₁₀(g)
The following known equations, determined by calorimetry, are provided: (4 marks)
(1) C₄H₁₀(g) + 13/2 O₂(g) → 4CO₂(g) + 5H₂O(g) ΔH° = –2657.4 kJ
(2) C(s) + O₂(g) → CO₂(g) ΔH° = –393.5 kJ
(3) 2H₂(g) + O₂(g) → 2H₂O(g) ΔH° = –483.6 kJ
*Given the following standard heats of formation, what is the ΔH° in kilojoules for the combustion of 1 mol of ethanol, C₂H₅OH(l), to form gaseous carbon dioxide and gaseous water, given the reaction:
C₂H₅OH(l) + 3 O₂(g) → 2 CO₂(g) + 3 H₂O(g) (3 marks)
Substance | ΔH°f (kJ/mol) |
|---|---|
H₂O(g) | –241.8 |
H₂O(l) | –286 |
CO₂(g) | –393.5 |
C₂H₅OH(l) | –235.2 |