topic 1 - key concepts in chemistry
1/96
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
97 Terms
describe how the dalton model of an atom has changed over time
the dalton model of an atom was a solid sphere containing no sub-atomic particles
as sub-atomic particles were discovered, they were added to the dalton model to become the ‘plum pudding model'
plum pudding model was a solid, positively charged sphere with negatively charged electrons interspersed throughout
rutherford model - over time, electrons were added to shells surrounding the proton and neutron nucleus
describe the structure of an atom
a positively-charged nucleus
containing protons and neutrons
surrounded by negatively-charged electrons
in shells
recall the relative charge of a proton
+1
recall the relative mass of a proton
1
recall the relative charge of a neutron
0
recall the relative mass of a neutron
1
recall the relative charge of an electron
-1
recall the relative mass of an electron
0.0005
explain why atoms contain equal numbers of electrons and protons
atoms have a neutral charge
an equal number of protons and electrons cancel out any net positive or negative charge
causing the atom to remain neutrally-charged
describe the size of the nucleus of an atom compared to the overall size of the atom
very small in comparison
recall where the majority of mass is concentrated in an atom
nucleus
state the meaning of mass number of an atom
the sum of the number of protons and neutrons in the nucleus
describe what different atoms of the same given element have
same number of protons in the nucleus
the number of protons in the nucleus is unique to the given element
describe what isotopes are
different atoms of the same element
containing the same number of protons
but a different number of neutrons in the nucleus
explain how the existence of isotopes results in relative atomic masses of some elements not being whole numbers
isotopes of the same element have the same number of protons but different numbers of neutrons to each other
this leads to different atomic masses with each isotope
when finding the mean average of these results, the relative atomic mass may appear as a decimal as this is the average of the isotope’s atomic masses
state how to calculate relative atomic mass of an element from given isotopes
(mass of i1 x abundance of i1) + (mass of i2 x abundance of i2) / 100
describe how mendeleev arranged the known elements at the time into a periodic table
columns - similar chemical properties of elements and their compounds
rows - increasing atomic mass
gaps - left gaps for undiscovered elements
exceptions - there were a few exceptions, like iodine, which didn’t fit the pattern by atomic mass
state why mendeleev did not use relative atomic mass in his periodic table
because isotopes had not yet been discovered, meaning relative atomic masses of elements could not be calculated
describe how mendeleev used his periodic table to predict the existence and properties of undiscovered elements
mendeleev used gaps in his table as place-holders for undiscovered elements
due to the location of these gaps within the table, mendeleev could calculate their general chemical properties and atomic masses
due to trends found within the columns and rows of the table
explain the issues with mendeleev’s table due to undiscovered isotopes
mendeleev thought he had organised the elements into correct ascending atomic mass within their rows
but calculating relative atomic mass was not taken into consideration as isotopes had not been discovered
leading to the final atomic mass being wrong
and the element ending up in the wrong space in the table
explain how elements are arranged by atomic number in the modern periodic table
the modern periodic table is organised in increasing consecutive atomic number
in periods
describe the organisation of the modern periodic table
rows (periods) - elements are arranged in increasing atomic number
columns (groups) - elements are arranged in groups of similar properties
state the definition of atomic number
number of protons in the nucleus of an atom
state the definition of atomic mass
number of protons and neutrons in the nucleus of an atom
state how to determine if an element is a metal based on its location in the periodic table
metal elements are located from the left to the end of the middle in the periodic table
state how to determine if an element is a non-metal based on its location in the periodic table
non-metal elements are located in the top right of the periodic table
explain why there is a division between metals and non-metals in the periodic table due to atomic structure (metals)
the metal elements further to the left of the table have less electrons in their outer shells
as you descend the groups of metals, the outer shell electrons become further away from the nucleus due to increasing atomic size
this causes an increase in reactivity as you descend the groups
making it more likely for the elements to lose electrons during a reaction
explain why there is a division between metals and non-metals in the periodic table due to atomic structure (non-metals)
the non-metal elements further to the right have more electrons in their outer shells
as you descend the groups of non-metals, there is a decrease in reactivity
making it more likely for the elements to gain or share electrons during a reaction
state the maximum number of electrons the first shell of an atom can hold
2
state the maximum number of electrons the second shell of an atom can hold
8
state the maximum number of electrons the third shell of an atom can hold
8
state the maximum number of electrons the fourth shell of an atom can hold
20
explain how to predict the electronic configurations of the first 20 elements in the modern periodic table
the notation of the electronic configuration of the first 20 elements in the periodic table adds up to the atomic number of the specific elements
e.g. boron - atomic number (5) - electronic configuration (2,3)
reminder: how to calculate electronic configuration using carbon
carbon has an atomic number of 6, meaning there are 6 protons and therefore 6 electrons
shells fill from the first shell to the third
2 of the electrons will be in the full first shell
4 of the electrons will be in the incomplete second shell
explain how the electronic configuration of an element is related to its position in the periodic table
the number of shells in the electronic configuration of an element is the same as the period it is in
e.g. elements in period 3 have 3 outer shells
the number of electrons in the outermost shell is the same as the group it is in
e.g. elements in group 7 have 7 elements in their outermost shell
describe the limitations of dot-and-cross diagrams in showing ionic bonding
don’t show the relative sizes of ions
don’t show ions are arranged
describe the limitations of ball-and-stick models in showing ionic bonding
suggests there are gaps between ions which is not true
describe the limitations of 2D representations in showing ionic bonding
doesn’t show the arrangement of ions in all layers
describe the limitations of 3D representations in showing ionic bonding
only shows the outer layer of ions
explain how ionic bonds are formed
transfer of electrons
between metal and non-metal atoms
to produce cations and anions
recall what an ion is
an atom or group of atoms
with a positive or negative charge
state how to calculate number of protons in an atom given its atomic number and mass
number of protons = atomic number
state how to calculate number of electrons in an atom given its atomic number and mass
number of electrons = atomic number
state how to calculate number of neutrons in an atom given its atomic number and mass
number of neutrons = atomic mass - atomic number
explain the formation of ions in ionic compounds in groups 1 and 2
atoms of elements in groups 1 and 2 will form cations
as they will lose electrons
due to these atoms having a lower number of electrons in their outermost shells
meaning it is easier to complete their outer shell by losing electrons
explain the formation of ions in ionic compounds in groups 6 and 7
atoms of elements in groups 6 and 7 will form anions
as they will gain electrons
due to these atoms having a higher number of electrons in their outermost shells
meaning it is easier to complete their outer shell by gaining electrons
resulting in an overall net negative charge
explain the use of the ending -ide in naming compounds
used when a compound contains only 2 atoms
explain the use of the ending -ate in naming compounds
used when a compound contains three or more atoms, one being oxygen
reminder: how to calculate the formulae of ionic compounds using the crossover rule (aluminium sulfate)
aluminium ion - Al³⁺
sulfate ion - SO₄²⁻
aluminium sulfate compound - Al₂(SO₄)₃
explain the structure of an ionic compound
giant lattice
regular arrangement of ions
held together between strong electrostatic forces of attraction
between oppositely-charged ions
explain why ionic compounds have high boiling and melting points
due to the large amount of energy needed
to overcome
strong electrostatic forces of attraction
between oppositely-charged ions
explain why ionic compounds don’t conduct as solids
ions are held in fixed positions
so they cannot move
and therefore cannot carry charge
explain why ionic compounds conduct when molten or in aqueous solutions
ions are not held in fixed positions
so they can move
and therefore can carry charge
state the formula of aluminium
Al³⁺
state the formula of ammonium
NH⁴⁺
state the formula of bromide
Br⁻
state the formula of calcium
Ca²⁺
state the formula of carbonate
CO₃²⁻
state the formula of chloride
Cl⁻
state the formula of fluoride
Fl⁻
state the formula of sodium
Na⁺
state the formula of hydroxide
OH⁻
state the formula of oxide
O²⁻
state the formula of iodide
I⁻
state the formula of phosphate
PO₄³⁻
state the formula of lithium
Li⁺
state the formula of strontium
Sr²⁺
state the formula of potassium
K⁺
state the formula of sulfide
S²⁻
state the formula of nitrate
NO₃⁻
state the formula of sulfate
SO₄²⁻
explain how a covalent bond is formed
a group of non-metal atoms
sharing pairs of electrons
to complete all atoms’ outer shells
recall the bonding used in the formation of molecules
covalent bonding
explain the formation of hydrogen as a simple covalent substance
one single shared pair of electrons
between two hydrogen atoms
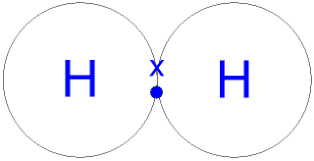
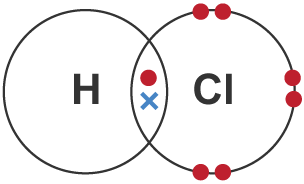
explain the formation of hydrogen chloride as a simple covalent substance
one single shared pair of electrons
between one hydrogen atom
and one chlorine atom
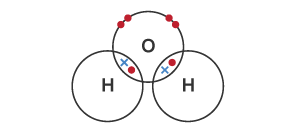
explain the formation of water as a simple covalent substance
two single shared pairs of electrons
between two hydrogen atoms
and one oxygen atom
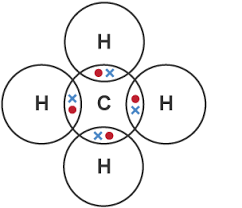
explain the formation of methane as a simple covalent substance
four single shared pairs of electrons
between four hydrogen atoms
and one carbon atom
explain the formation of oxygen as a simple covalent substance
one double shared pair of electrons
between two oxygen atoms
explain the formation of carbon dioxide as a simple covalent substance
two double shared pair of electrons
between two oxygen atoms
and one carbon atom
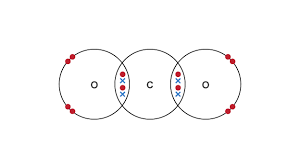
explain why simple covalent compounds have low melting/boiling points
contain weak intermolecular forces between atoms
which need low amounts of energy to overcome
explain why simple covalent compounds are poor conductors of electricity
they contain no charged particles
which therefore cannot move and carry charge
recall what type of covalent substance graphite is
giant covalent substance
recall what type of covalent substance diamond is
giant covalent substance
describe the structure of graphite
each carbon atom is joined to three other carbon atoms by covalent bonding and one delocalised electron
carbon atoms form hexagonal layered structure
layers have weak intermolecular forces between them, allowing them to slide over each other
describe the structure of diamond
each carbon atom is joined to four other carbon atoms by covalent bonding
carbon atoms form a regular tetrahedral structure
no free electrons
explain why graphite is used to make electrodes
graphite contains a sea of delocalised electrons
which are capable of moving and carrying charge
allowing them to conduct electricity
making it a good material for electrodes
explain why graphite is used as a lubricant
graphite contains weak intermolecular forces between hexagonal layers
allowing the layers to slide over each other
making graphite a soft material and good to use as a lubricant
explain why diamond is used in cutting tools
each carbon atom is joined to four other carbon atoms by covalent bonding
giving diamond a rigid tetrahedal structure
the rigidity allows diamond to be a good material for cutting tools
state the properties of buckminsterfullerene
low melting point
slippery
explain why buckminsterfullerene has a low melting point
low amount of energy needed
to overcome weak intermolecular forces
between buckminsterfullerene compounds
explain why buckminsterfullerene is slippery
weak intermolecular forces
between buckminsterfullerene layers
allowing the layers to slide over each other
state the formula of buckminsterfullerene
C₆₀
state the properties of graphene
strong
high melting point
lightweight
thermal and electrical conductor
explain why graphene is strong
strong covalent bonds between carbon atoms
explain why graphene is lightweight
graphene is a single layer of graphite
explain why graphene has a high melting point
large amount of energy needed to overcome
strong covalent bonds
between carbon atoms
explain why graphene is a conductor
contains a sea of delocalised electrons
which are capable of moving and carrying charge