PAG 6 - Hydrolysis of an ester
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What acid does the hydrolysis of methyl benzoate make?
Benzoic acid
Complete the following equation and give the symbols:
Methyl benzoate + sodium hydroxide
Methyl benzoate + sodium hydroxide → sodium benzoate + methanol
C6H5COOCH3 + NaOH → C6H5COO-Na+ + CH3OH
Give the method for reacting methyl benzoate with sodium hydroxide
Pour methyl benzoate into a round bottomed flask
Add equal amounts of sodium hydroxide solution and ethanol to flask
Add a few anti-bumping granules and set equipment up for refluxing
Reflux for 20 minutes
Allow the contents of the flask to cool with the condenser still in place. When cool, remove condenser from flask and decant into a beaker.
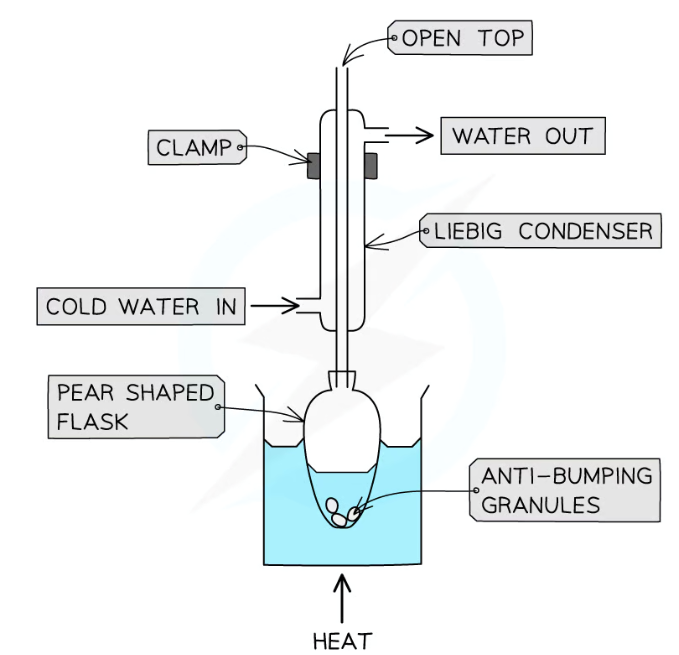
Give the reaction for the formation of benzoic acid
C6H5COO- + H+ → C6H5COOH
Give the method for the production of benzoic acid from the mixture above.
Add 4-5 drops of methyl orange and stir into the solution
Acidify with small portions of hydrochloric acid, stirring after each addition
Filter the solid product under reduced pressure
Recrystallise the product (benzoic acid) using a minimum volume of boiling water
Filter the purified benzoic acid under reduced pressure and allow to dry in the air
After the reflux, what observations are made when adding the HCl portions?
Methyl orange changes to pink/red colour and a white solid forms, indicating an acid
Why is water used as a solvent for recrystallisation?
Water is polar and can dissolve the benzoic acid
Why is the first solution acidified with HCl?
Adding H+ ions allows the formation of benzoic acid
Suggest a significant procedural error and a modification which could be made
The use of an indicator as it means an extra impurity which we must get rid of
We could instead do one experiment with indicator to work out amount of acid needed, then repeat without indicator
Or we could use a pH probe