Liver Function
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
Liver General:
Functions & Resiliancy
Large & Complex Organ
Function:
Metabolism of carbs, lipids, proteins, bilirubin
Detox of harmful subs.
Storage of essential compounds
Clearance of waste products into bile or blood for extraction
Resilient
Can regenerate with short term damage
regeneration is limited
Gross Liver Anatomy Overview
Weight: 1.2–1.5 kg in healthy adult
Location: Beneath & attached to diaphragm, protected by ribs
Support: Held in place by ligaments (e.g., falciform ligament)
Lobes: Right (larger), Left (smaller)
Adjacent organs:
Gallbladder under right lobe
Stomach near left lobe
Key anatomical relation: Lies directly below diaphragm, superior to most abdominal organs
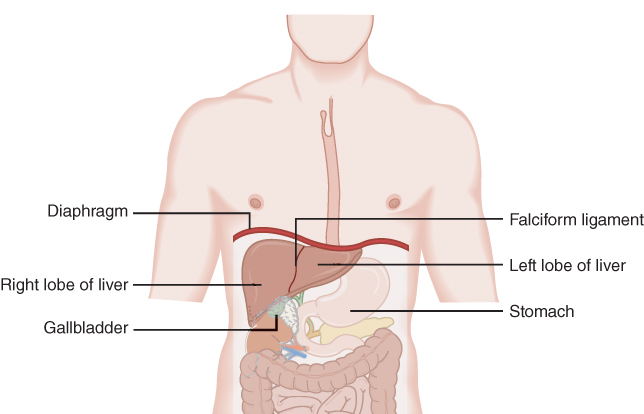
Liver Vasculature & Lobular Structure
Highly vascularized organ
Two lobes:
Connected by falciform ligament
Right lobe is ~6× larger than left
Dual blood supply:
Hepatic artery (25%) – oxygenated blood
Portal vein (75%) – nutrient-rich blood from GI tract
~1500 mL/min blood flow through liver
Despite size difference, lobes are functionally equal
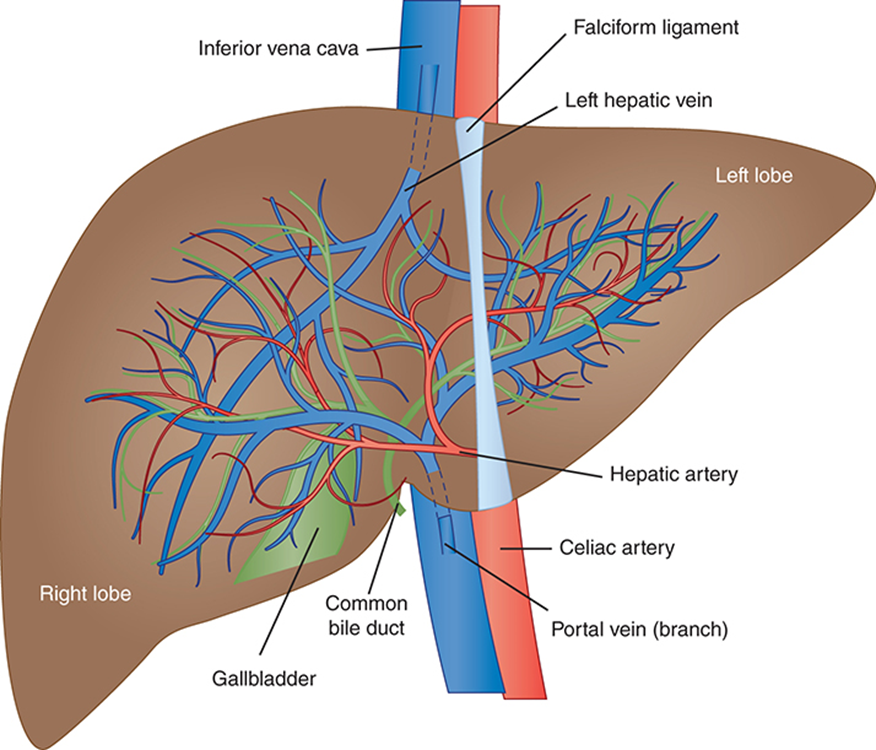
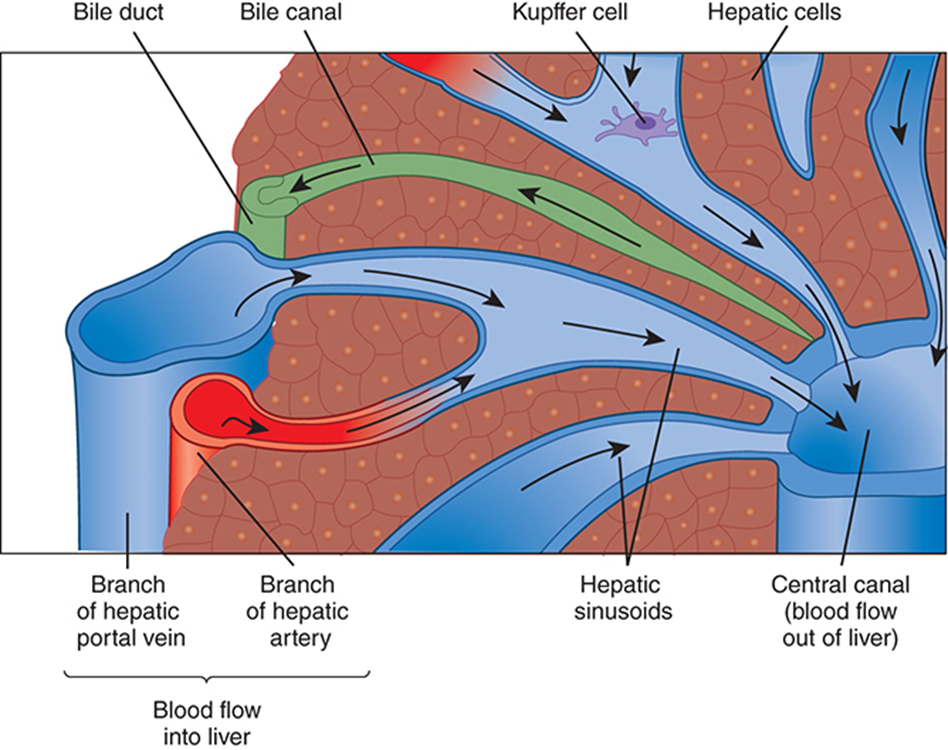
Bile Flow in the Hepatic Excretory System
Begins in bile canaliculi (tiny channels between hepatocytes)
Canaliculi → merge into right & left hepatic ducts
Hepatic ducts → join to form the common hepatic duct
Cystic duct from gallbladder joins → forms common bile duct
Common bile duct empties into the duodenum
Function: carries excretory products (e.g., bile) for digestion & waste removal
Hepatic Lobules & Liver Cell Types
Liver divided into microscopic lobules
Hexagonal units with a central vein
Portal triads at each corner (portal vein, artery, bile duct)
Perform all excretory & metabolic functions
Blood flows inward to central vein; bile flows outward to bile ducts
Major cell types:
Hepatocytes (~80%) → metabolic workhorses
Kupffer cells → liver macrophages; phagocytose debris & toxins in sinusoids
Core Biochemical Roles of the Liver
4 essential functions:
• Excretion/Secretion – bile, bilirubin, hormones
• Storage – glycogen, vitamins (A, D, B12), iron
• Metabolism – carbs, fats, proteins, drug processing
• Detoxification – ammonia → urea, toxins, alcoholVital for glucose homeostasis:
• Liver maintains blood glucose via glycogenolysis and gluconeogenesis
• If liver fails → severe hypoglycemia → coma/deathLiver failure = fatal within 24 hours if glucose regulation ceases
Hepatic Excretion & Bile Formation
Liver excretes endogenous & exogenous waste via bile or urine
• Example: bilirubin from heme catabolismOnly organ that can eliminate heme waste
Bile composition:
• Bile salts, bile pigments, cholesterol, and other extracted substancesLiver produces ~3 L/day of bile, excretes ~1 L/day
Bilirubin = primary bile pigment
Bilirubin Production Pathway
RBC lifespan ≈ 120 days
Aged RBCs are broken down, releasing hemoglobin
Hemoglobin → heme, globin, and iron
• Globin → amino acids (recycled)
• Iron → bound to transferrin, sent to liver or marrowHeme → converted to bilirubin within 2–3 hrs
Bilirubin → bound to albumin → transported to liver
Bilirubin Metabolism
RBCs break down → heme → bilirubin
Bilirubin binds albumin → travels to liver
In liver:
• Unconjugated bilirubin (water-insoluble)
→ becomes conjugated via UDP-glucuronyl transferase (water-soluble)Conjugated bilirubin → secreted into bile → intestines
• Gut bacteria convert it to urobilinogenFate of urobilinogen:
• Majority → feces (gives stool brown color)
• Some → reabsorbed → urine (1–4 mg/day)
Liver’s Role in Carbohydrate Metabolism
One of liver’s most vital functions
3 ways liver processes glucose:
• Uses it for own energy needs
• Releases glucose to peripheral tissues
• Stores it as glycogenMaintains blood glucose homeostasis via:
• Glycogenesis – stores glucose as glycogen
• Glycogenolysis – breaks down glycogen to glucose
• Gluconeogenesis – makes glucose from non-carb precursors (e.g. amino acids)
Hepatic Lipid Metabolism Essentials
Liver metabolizes lipids & lipoproteins under normal conditions
Free fatty acids from diet → converted to acetyl-CoA
Acetyl-CoA used to synthesize:
• Triglycerides
• Phospholipids
• Cholesterol~70% of daily lipid production comes from the liver, not the diet
Liver Functions in Protein Metabolism
Almost all proteins are synthesized in the liver
• Exception: immunoglobulins and adult hemoglobinLiver is essential for Hgb synthesis in infants
Albumin = key plasma protein for oncotic pressure & transport
Synthesizes:
• Acute-phase reactants
• Coagulation proteins
• Amino acid pool storageCritical metabolic roles:
• Transamination – transfers amino groups
• Deamination – removes amino groups for energy use or urea cycle
Liver Detoxification & Drug Metabolism
Filters waste from physiological processes (e.g. bilirubin, ammonia)
Acts as gatekeeper: filters substances absorbed in GI tract before systemic release
First-pass metabolism: all GI-absorbed substances pass through liver first
→ protects body from toxins/drugs entering circulationTwo detox strategies:
• Bind & inactivate compound
• Chemically modify it for excretionDrug metabolism pathways:
• Oxidation, reduction, hydrolysis, hydroxylation, carboxylation, demethylation
Liver Dysfunction in Disease States
Jaundice – buildup of bilirubin (pre-, intra-, or post-hepatic causes)
Cirrhosis – chronic liver damage → fibrosis, nodules, impaired function
Tumors – hepatocellular carcinoma or metastatic lesions disrupt function
Reye’s Syndrome – rare, acute liver failure (often post-viral in children)
Drug & Alcohol Disorders – cause fatty liver, hepatitis, fibrosis, cirrhosis
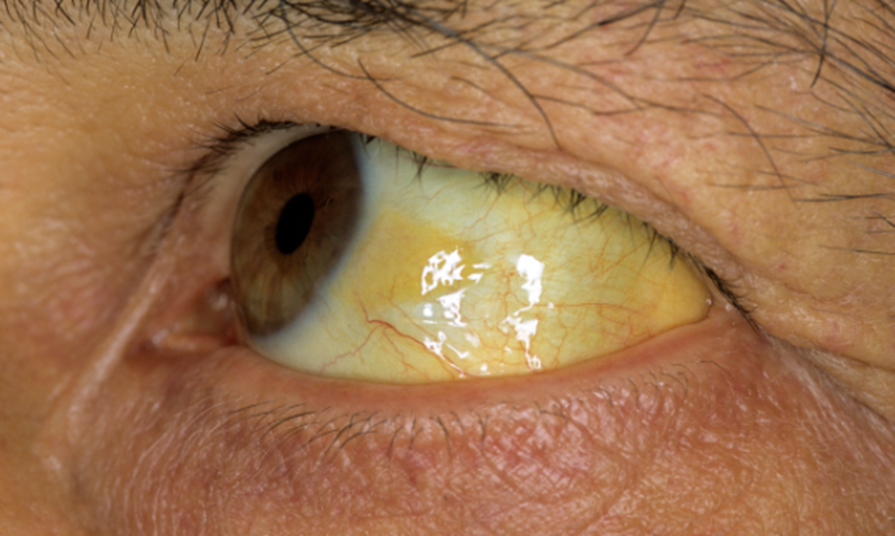
Jaundice – Clinical Presentation & Thresholds
Definition: Yellow discoloration of skin, sclera, mucous membranes
Caused by retention of bilirubin in tissues
Normal total bilirubin: 1.0–1.5 mg/dL
Visible jaundice not seen until levels reach 3.0–5.0 mg/dL
Icterus & Jaundice Classification by Origin
Jaundice is classified by site of dysfunction:
• Prehepatic – excess RBC destruction → ↑ unconjugated bilirubin
• Hepatic – impaired conjugation or hepatocellular injury
• Posthepatic – bile duct obstruction → ↑ conjugated bilirubin buildup

Prehepatic (Unconjugated) Jaundice
Caused by excess bilirubin production → overwhelms liver
• Common in acute/chronic hemolytic anemiasCharacterized by unconjugated hyperbilirubinemia
• Bound to albumin, not excreted in urineBilirubin levels rarely exceed 5.0 mg/dL
Posthepatic (Obstructive) Jaundice
Caused by biliary obstruction (e.g. gallstones, tumors)
Conjugated bilirubin is formed but can’t be excreted into bile
Bile flow blocked → no bilirubin enters intestines
• Results in clay-colored stool due to absence of urobilinogen
Hepatic Jaundice – Intrinsic Liver Dysfunction
Primary defect is in the liver itself
Caused by:
• Bilirubin metabolism or transport defects
• Hepatocellular injury or destruction (e.g. hepatitis, toxins, cirrhosis)Leads to mixed elevation of conjugated & unconjugated bilirubin
Gilbert’s Syndrome
Benign autosomal recessive disorder (~5% of U.S. population)
No morbidity, mortality, or clinical consequences
Intermittent unconjugated hyperbilirubinemia
• Due to reduced conjugation activity (≈30% of normal function)Total bilirubin typically 1.5–3.0 mg/dL, rarely exceeds 4.5 mg/dL
Crigler-Najjar Syndrome
Inherited disorder causing chronic, nonhemolytic unconjugated hyperbilirubinemia
Caused by defective bilirubin conjugation enzyme
Type 1: complete absence of enzyme → fatal without treatment
Type 2: severe enzyme deficiency → milder but still dangerous
Rare; may lead to kernicterus or death without proper managemen
Dubin-Johnson Syndrome
Conjugated hyperbilirubinemia
Rare autosomal recessive disorder, obstructive in nature
Impaired excretion of conjugated bilirubin → buildup of delta bilirubin (bound to albumin)
Dark-stained liver granules seen on biopsy
Total bilirubin: 2–5 mg/dL
No treatment needed, normal life expectancy
Rotor Syndrome
Autosomal recessive liver disorder
Rare & benign; no major clinical consequences
Impaired hepatic uptake and clearance of bilirubin
Labs: normal ALP and GGT → helps distinguish from biliary obstruction
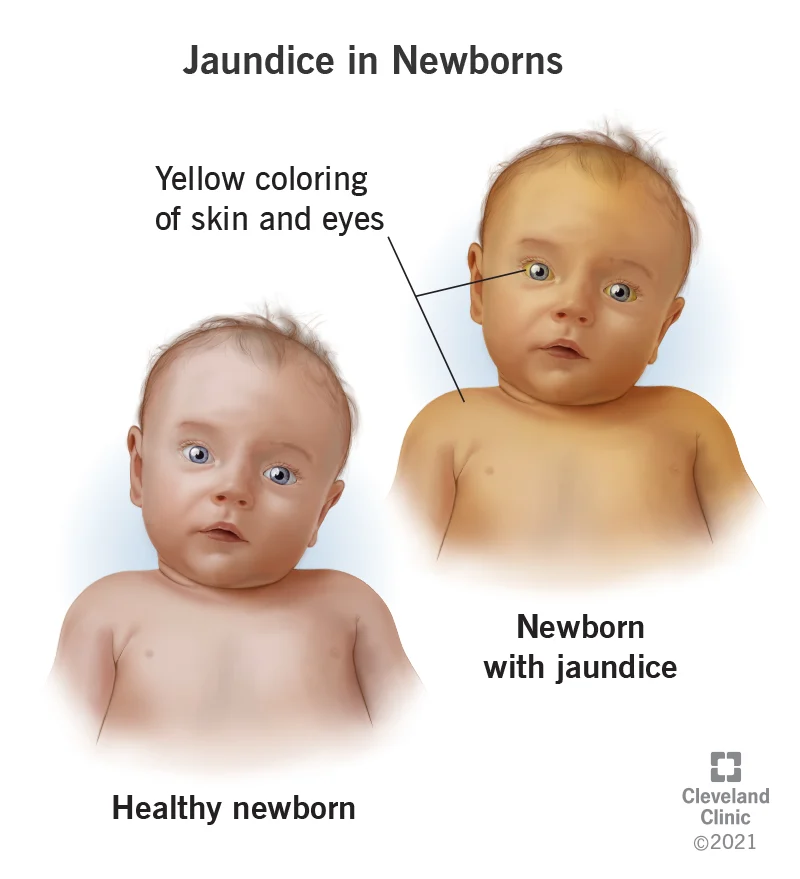
Physiologic Jaundice
NEONATAL HYPERBILIRUBINEMIA
Seen in newborns, typically in the first week of life
Caused by immature liver and deficiency of UDP-glucuronyl transferase (UDPGT)
Unconjugated bilirubin builds up; premature infants at higher risk
Can progress to kernicterus (bilirubin deposition in brain) → brain damage/death
Dangerous levels: can exceed 20 mg/dL
Treatment:
• Phototherapy
• Exchange transfusion
• Pharmacologic therapy, IVIG, metalloporphyrins
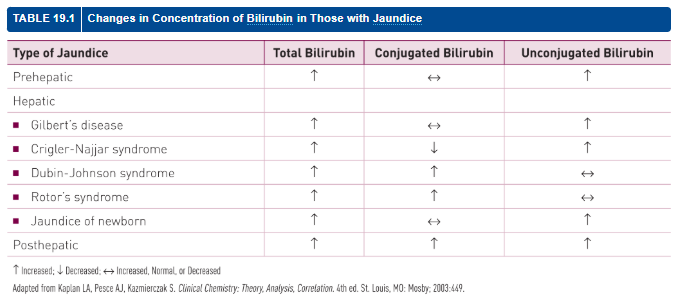
Bilirubin Patterns in Jaundice Disorders
Condition | Total Bili | Conjugated | Unconjugated |
|---|
Prehepatic | ↑ | ↔ | ↑ |
Gilbert’s Syndrome | ↑ | ↔ | ↑ |
Crigler-Najjar Syndrome | ↑ | ↓ | ↑ |
Dubin-Johnson Syndrome | ↑ | ↑ | ↔ |
Rotor Syndrome | ↑ | ↑ | ↔ |
Jaundice of Newborn | ↑ | ↔ | ↑ |
Posthepatic | ↑ | ↑ | ↑ |
Cirrhosis
Chronic liver disease → replacement of healthy tissue with scar tissue
• Disrupts blood flow and normal liver functionOften asymptomatic early; late signs: fatigue, jaundice, GI bleeding, ascites, pruritus
Poor prognosis once advanced
Most common cause: alcoholism
• Treatment: abstinenceOther causes: viral hepatitis (HBV, HCV, HDV), autoimmune hepatitis, NAFLD, toxins
Treatment may include: interferons, corticosteroids
Liver Tumors
Majority (90–95%) are metastatic from colon, lung, or breast
Benign types: hepatocellular adenomas, hemangiomas
Malignant types:
• Hepatocellular carcinoma (HCC) – 90% of primary liver cancers
• Bile duct carcinomaHCC risk factors: cirrhosis, HBV/HCV, heavy alcohol use, NAFLD, mycotoxins, smoking
Malignant tumors have poor prognosis; survival measured in months
Reye’s Syndrome
Pediatric disorder, often post-viral, with aspirin association
Characterized by:
• Noninflammatory encephalopathy
• Fatty liver degeneration
• Profuse vomiting, neurologic symptomsLabs:
• Mild hyperbilirubinemia
• ↑↑ ammonia, AST, ALTUntreated → rapid deterioration and possible death
Drug-Induced Liver Disease
Accounts for 1/3–1/2 of acute liver failure cases in the U.S.
Liver is a central site of drug metabolism → highly susceptible to injury
Caused by many medications, with severity ranging from mild to fatal
Most common mechanism:
• Immune-mediated hepatocyte injury
Alcohol-Related Liver Disease
Ethanol is the leading hepatotoxic drug
90% of alcohol is metabolized by the liver
Low doses: mild, often unnoticed injury
Chronic use → progression through 3 stages:
• Alcoholic fatty liver
• Alcoholic hepatitis
• Alcoholic cirrhosisSeverity and progression vary; exact toxic threshold is unknown
Fatty Liver Disease
Typically very mild with few functional changes
Slight increases in liver enzymes: AST, ALT, GGT
Biopsy: fatty infiltration confirmed
Common in middle-aged adults with:
• Obesity, diabetes, moderate alcohol useTreatment: risk factor reduction, diet & exercise, diabetes control, limit alcohol
Alcoholic Hepatitis
Symptoms: fever, ascites, muscle wasting, abnormal labs
Labs:
• Moderate elevation in AST, ALT, GGT, ALP
• Total bilirubin often >5 mg/dL
• Low serum albumin, ↑ prothrombin time
• Rising creatinine = poor prognosisSeverity and outcome depend on extent of liver damage
Alcoholic Cirrhosis
Prognosis depends on complications (e.g. GI bleed, ascites)
5-year survival:
• 60% with abstinence
• 30% with continued drinkingSymptoms: weight loss, weakness, hepatosplenomegaly, jaundice, ascites, edema
Labs:
• ↑ AST, ALT, GGT, ALP, bilirubin
• ↓ albumin, prolonged prothrombin timeLiver biopsy confirms diagnosis
Other Hepatotoxic Drugs
Liver injury can range from mild to fatal failure or cirrhosis
Risk drugs include:
• Tranquilizers, some antibiotics
• Antineoplastic agents, lipid-lowering drugs, anti-inflammatoriesAcetaminophen:
• In large doses, causes fatal hepatic necrosis without rapid intervention
Bilirubin Testing History
Based on the diazo reaction (Ehrlich, 1883); modern tests are modified versions
Van den Bergh (1937) added accelerators to measure direct bilirubin
Today:
• Total and direct bilirubin measured
• Indirect bilirubin is calculatedNeonates: use bilirubinometry (light reflectance via skin)
Bilirubin types:
• Unconjugated (indirect), conjugated (direct), and delta (albumin-bound)
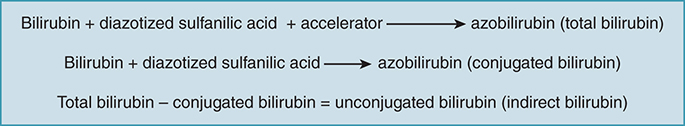
Bilirubin Fraction Determination (Diazo Method)
Total bilirubin:
• Bilirubin + diazotized sulfanilic acid + accelerator → azobilirubinConjugated (direct) bilirubin:
• Bilirubin + diazotized sulfanilic acid → azobilirubinUnconjugated (indirect) bilirubin:
• Total bilirubin – conjugated bilirubin
Bilirubin Collection and Handling
Use serum or plasma
• Malloy-Evelyn method: prefers serum (avoids protein interference from alcohol)Fasting preferred: reduces lipemic interference
Avoid hemolysis: hemoglobin inhibits diazo reaction
Protect from light:
• Exposure may cause 30–50% bilirubin loss per hour
Malloy-Evelyn Method
Bilirubin reacts with diazotized sulfanilic acid
Performed at pH 1.2
Produces azobilirubin (red-purple, max absorbance at 560 nm)
Methanol is the most common accelerator
Less stable; light-sensitive reagent
endrassik-Grof Method
Bilirubin reacts with diazo reagent in two aliquots
• Diazo only → conjugated bilirubin
• + caffeine-benzoate → total bilirubinWidely used (modified forms common)
Advantages:
• Not affected by pH or protein variation
• High sensitivity, even at low bili
• Minimal turbidity, constant serum blank
• Tolerates hemoglobin up to 750 mg/dL
Adult Bilirubin Reference Ranges
Direct (conjugated) bilirubin:
• 0.0–0.2 mg/dLIndirect (unconjugated) bilirubin:
• 0.2–0.8 mg/dLTotal bilirubin:
• 0.2–1.0 mg/dL
Urobilinogen
Colorless end product of bilirubin metabolism
Fate in body:
Some excreted in feces
Remainder reabsorbed → portal blood → liver
Small portion excreted by kidneys as urobilinogen
In feces: oxidized by bacteria → brown pigment urobilin
Clinical associations:
↑ Urinary urobilinogen: hemolytic disease, hepatitis
↓/Absent urobilinogen: complete biliary obstruction
Urobilinogen Testing
Based on reaction w/ Ehrlich's reagent (p-dimethylaminobenzaldehyde)
Forms red color
Typically assessed in urinalysis, not chemistry
Liver Function Enzymes
Used to assess liver injury and function
Injury (e.g., cytolysis or necrosis) → enzymes released into circulation
Enzymes help differentiate:
Hepatocellular (functional) vs. Obstructive (mechanical) damage
Most useful LFTs:
ALT, AST (aminotransferases)
ALP, 5′-nucleotidase (phosphatases)
GGT
LD (lactate dehydrogenase)
Aminotransferases (ALT & AST)
ALT = Liver-specific, rises more than AST, remains elevated 2–6 wks
→ ALT = “L” for Long & LiverAST = Found in heart, skeletal muscle, and liver
Highest elevations seen in:
Acute viral hepatitis
Drug/toxin-induced necrosis
Hepatic ischemia
Moderate elevations in less severe disease
Present in multiple tissues → interpret cautiously
Serial testing recommended—levels may drop in severe necrosis from depletion of hepatocellular stores
Alkaline Phosphatase (ALP)
Widely distributed: highest in liver, bone, intestine, kidney, placenta
Differentiates hepatobiliary disease vs osteogenic bone disease
Very high ALP → extrahepatic biliary obstruction
Slight to moderate elevation → hepatocellular disorders (e.g., hepatitis, cirrhosis)
Gamma-Glutamyl Transferase (GGT)
Differentiates elevated ALP from skeletal vs hepatobiliary sources
Highest levels in biliary obstruction
Most sensitive hepatic enzyme indicator for liver disease
Elevated by alcohol, enzyme-inducing drugs, and cholestasis
Helpful when jaundice is absent to confirm hepatic neoplasms
Lactate Dehydrogenase (LD)
Widely distributed throughout body
Released with cell damage or destruction
Acts as a nonspecific marker of injury
Moderate ↑: acute viral hepatitis, cirrhosis
Slight ↑: biliary tract disease
High ↑: metastatic carcinoma of the liver
5′-Nucleotidase (5NT)
Rarely ordered; performed at large reference labs
No bone source → helpful with ALP to isolate liver origin
↑ in hepatobiliary disease
More sensitive to metastatic liver disease
Tests of Hepatic Synthetic Ability
Evaluates serum proteins to assess liver’s synthetic function
↓ Albumin → decreased protein synthesis
↓ Alpha-globulins in chronic liver disease
↑ Gamma-globulins in acute/chronic liver disease
IgG, IgM in chronic active hepatitis
IgM ↑ in primary biliary cirrhosis
IgA ↑ in alcoholic cirrhosis
↑ Prothrombin Time (PT) = poor prognosis, severe liver dysfunction
Tests Measuring Nitrogen Metabolism
Liver clears ammonia from bloodstream by converting it to urea
Ammonia levels reflect liver function
↑ Ammonia/toxins may cause hepatic coma
(note: severity of symptoms doesn't always match ammonia level)Ammonia is unstable—must be kept on ice to prevent metabolism
Hepatitis
Definition: Inflammatory condition of the liver
Causes:
Non-infectious: Radiation, chemicals, autoimmune disease, toxins
Infectious:
Viral (most common): HAV, HBV, HCV, HDV, HEV
Bacterial, parasitic
Acute symptoms: Jaundice, dark urine, fatigue, nausea, vomiting, abdominal pain
Chronic hepatitis:
Transaminase elevation >6 months
Common with HBV and HCV
HAV - Key Features
Aka infectious hepatitis or short incubation
Most common form worldwide (~1.5 million cases annually)
Caused by non-enveloped RNA virus (Picornavirus)
Transmission: fecal-oral route (via food, water)
Excreted in bile and shed in feces
HAV - Symptoms & Prognosis
Symptoms: fever, malaise, anorexia, nausea, abdominal discomfort, dark urine, jaundice
Self-limiting: resolves in ~3 weeks
Liver failure is rare
No chronic infection or carrier state
HAV - Vaccination
Vaccines available: offer long-term immunity
Recommended for children and travelers to endemic areas
HAV - Diagnosis: Serology
IgM anti-HAV:
Appears early; detects acute infection
Detectable at/just before symptoms
Disappears by 3–6 months
IgG anti-HAV:
Appears after IgM
Persists for life = immunity
IgM–, IgG+ = past infection
HAV - Diagnosis: Other Methods
Fecal antigen detection during acute phase
Disappears after liver enzymes peak
RT-PCR:
More sensitive than immunoassay
Useful for asymptomatic cases
Works with various sample types
HCV – Clinical Features & Transmission
RNA virus, parenterally transmitted (blood products, IV drug use).
Leading cause of liver transplants in the U.S.
~2.5% of world infected; ~2.4 million U.S. cases.
Often asymptomatic acutely, but 80% become chronic.
High risk of progression to cirrhosis, carcinoma.
No vaccine available; treatment via antiviral meds.HCV
HCV – Testing & Monitoring
Initial screen: anti-HCV antibody (EIA).
If positive → confirm with HCV RNA (PCR).
Anti-HCV not detectable in early infection window.
Chronic cases monitored by RNA viral load.
Vaccine unlikely due to viral variability.
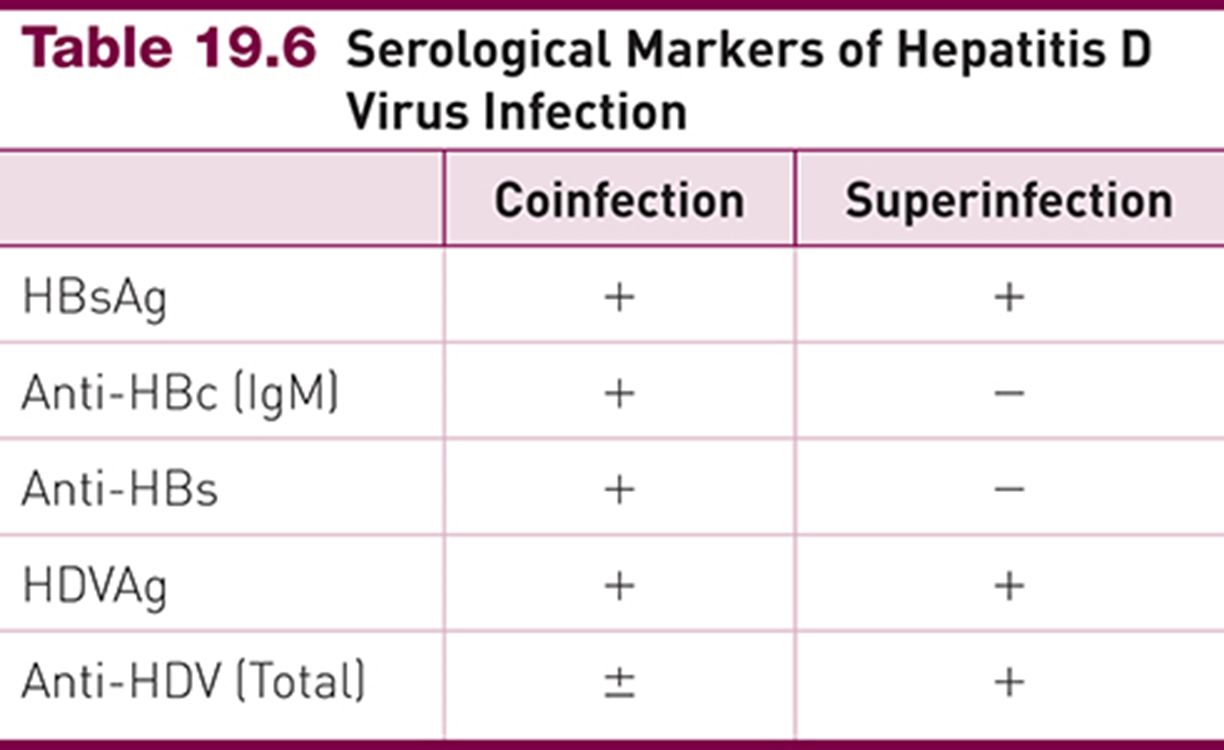
Hepatitis D
Small, defective RNA virus
Requires HBsAg from HBV to replicate → can't infect alone
Must also have HBV to get HDV infection
Coinfection: HDV + HBV at same time
Superinfection: HDV after chronic HBV → more severe
~5% of global HBV carriers have HDV
Serologic patterns (coinfection vs. superinfection):
Coinfection: HBsAg+, Anti-HBc IgM+, HDVAg+, Anti-HDV ±
Superinfection: HBsAg+, Anti-HBc IgM–, HDVAg+, Anti-HDV+
Hepatitis E
Fecal-oral transmission; waterborne outbreaks
Incubation: 21–42 days
Virus detected in feces ~7 days post-infection
Zoonotic potential: pigs, cows, sheep are reservoirs
Clinically resembles HAV
Diagnosed by anti-HEV IgM
No vaccine available
Other Forms of Hepatitis
Hepatitis F: Enteric agent; transmissible to primates
GB virus C (Hep G): Flaviviridae; infects humans, no disease
No diagnostic tests available
Other viral causes:
Cytomegalovirus (CMV)
Epstein-Barr virus (EBV)
SARS-CoV-2
Global Burden and Symptoms of HBV
AKA serum hepatitis or "long incubation" hepatitis
Can be acute or chronic
Most ubiquitous hepatitis virus
~2 billion infected globally
1.5 million new cases annually
U.S. stats:
~12 million infected
Peak incidence: ages 25–45
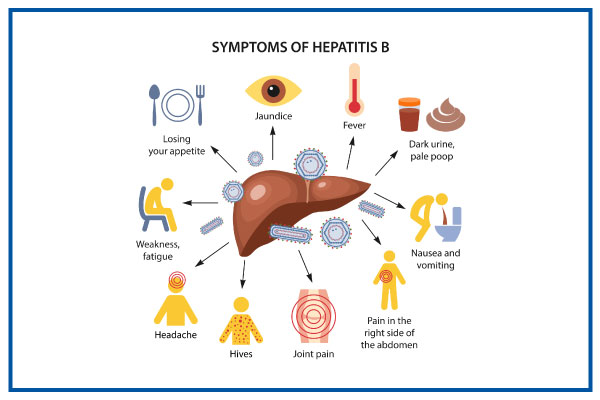
Common symptoms:
Jaundice, fever, dark urine, pale stool
Fatigue, nausea, abdominal pain (right abdominal)
Anorexia, joint pain, headache, hives
Transmission Routes and Environmental Stability
Survives >7 days on surfaces
Present in all body fluids, including:
Blood, feces, urine, saliva, semen, tears, breast milk
Transmission modes:
Parenteral (e.g., needle sharing, medical exposure)
Perinatal (mother-to-child at birth)
Sexual contact
Populations at High Risk for HBV Infection
High-risk behaviors:
Unprotected sex
Needle sharing
Perinatal transmission:
Infants born to HBsAg+ mothers
Geographic risk:
Immigrants from endemic areas
Household & sexual contacts of infected persons
Blood transfusions:
Risk is rare, but still possible
HBV Structure and Antigens
HBV is a DNA virus
Liver = primary site of viral replication
Infection process:
Antigen core synthesized in hepatocyte nuclei
Moves to cytoplasm
Surrounded by a protein coat
Key antigens:
HBcAg: core antigen, found inside virus
HBsAg: surface antigen, located on outer envelope
HBeAg: secreted antigen, linked to viral replication and infectivity
HBsAg – Hepatitis B Surface Antigen
Detected before symptoms appear
Used to screen all donated blood units
Indicates presence of HBV, but is not itself infectious
Chronic carriers of HBsAg = potentially infectious
Cannot rule out intact virus
Antibody to HBsAg develops after complete viral clearance
HBsAg – Detection and Diagnostic Window
First serologic marker to appear after HBV infection
Detected in 3–5 weeks
Average time to detection:
30 days post-exposure (range: 6–60 days)
Nucleic acid tests can detect HBV DNA
Positive 10–20 days before HBsAg
May be transiently positive 10–20 days post-vaccination
Anti-HBs – Hepatitis B Surface Antibody
Indicates past infection or successful vaccination
Appears after HBsAg clearance
Confers immunity to future HBV infection
Common in general population due to prior exposure or immunization
Used to assess:
Recovery from natural infection
Response to vaccination
Anti-HBs – Interpretation of Results
Positive result:
Indicates recovery from acute or chronic HBV
Reflects acquired immunity
Negative result:
Suggests no recovery from infection
May indicate inadequate immune response
HBcAg – Hepatitis B Core Antigen
Not detectable in blood—no test available for patients or donors
Intracellular only: found in hepatocyte nuclei during acute infection
Anti-HBc (core antibody) develops before Anti-HBs
Anti-HBc, Total – Interpretation and Utility
Detects past or chronic HBV infection
Appears during “window period” when HBsAg and Anti-HBs are negative
Useful in distinguishing recent vs chronic infection (w/ IgM status)
Interpretation summary:
Anti-HBc Total (+) → past or chronic infection
Anti-HBc IgM (+) → acute or recent infection
Anti-HBc IgM (–) → past or chronic infection
Anti-HBc Total (–) → no evidence of recent, past, or chronic infection
Anti-HBc Total (inconclusive) → possible interfering substances
HBsAg (+) with Anti-HBc Total (+) → chronic infection
Anti-HBc IgM – Marker of Acute HBV Infection
Specific for acute HBV infection
May persist at low levels in chronic HBV
Can be reactivated (test positive) during exacerbation of chronic HBV
Hepatitis B Envelope Antigen
Found in serum during acute or chronic HBV
Closely linked to the viral core
Correlates with:
Viral replication
Number of infectious particles
Infectivity of serum
Anti-HBe formation → low infectivity
HBeAg + HBsAg → poor prognosis, predicts chronic infection and severe disease course
Bridges structure with prognosis
Anti-HBe – Hepatitis B Envelope Antibody
Appears as HBeAg declines during recovery from acute infection
Remains detectable for several years
In chronic infection:
Low levels signal low infectivity and reduced transmission risk
Contextual note: Complements HBeAg—marks transition from high to low infectivity
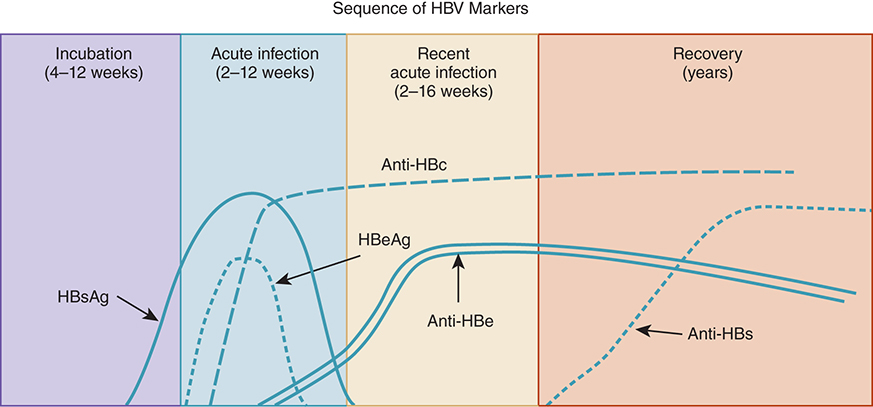
HBV Serology Timeline – Recovery Pattern
HBsAg appears first, peaks during acute illness
HBeAg follows, indicating infectivity
Anti-HBc IgM signals recent infection
Anti-HBe emerges as HBeAg declines
Anti-HBs appears after clearance of HBsAg → immunity
Window period: Anti-HBc may be the only positive marker
Contextual note: Diagram shows acute → resolved infection with sequential seroconversion.
Just know general trends
HBV Serology Timeline – Chronic Infection Patterns
HBsAg persists >6 months → chronic HBV
HBeAg may persist (poor prognosis) or decline with Anti-HBe formation (better outcome)
No Anti-HBs formed in chronic infection
Anti-HBc (total) stays positive regardless of chronicity
Just know general trends
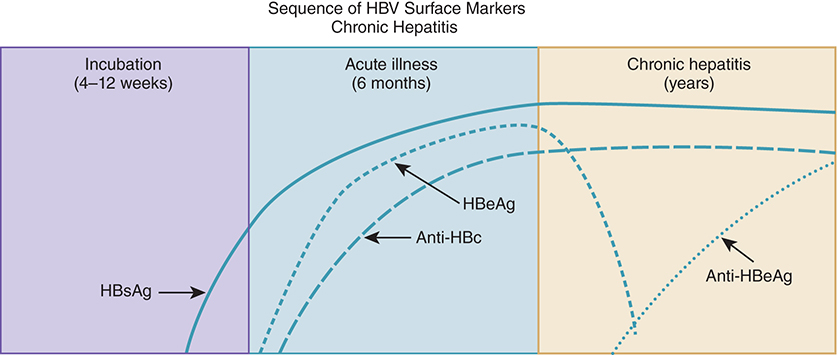
Chronic Hepatitis B – Outcomes and Risk Factors
90% of patients recover within 6 months (develop Anti-HBs)
10% develop chronic hepatitis:
Risk highest with perinatal infection (90%)
20–30% risk with infection during childhood
Among chronically infected:
25% infected since childhood and
15% infected as adults will die prematurely from cirrhosis or liver cancer
Phases of Chronic Hepatitis B Infection
Immune Tolerance:
Virus present, but immune system unresponsive
Immune Clearance:
Active immune response, HBeAg+, liver inflammation
Inactive Carrier State:
HBsAg+, but low/absent viral replication
Reactivation:
Return of viral replication, often with HBeAg reappearance
Indicates chronic active hepatitis
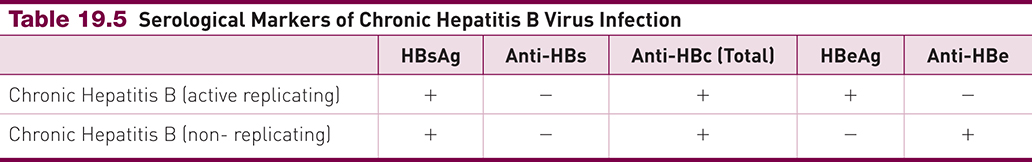
Chronic HBV – Active vs Inactive Serologic Profiles
Marker | Active Replicating | Non-Replicating |
|---|---|---|
HBsAg | + | + |
Anti-HBs | – | – |
Anti-HBc | + | + |
HBeAg | + | – |
Anti-HBe | – | + |
Contextual note: Use this profile to distinguish chronic active infection (high infectivity) from inactive carrier state—ties directly to the 4-phase model and timeline diagrams.
NEED TO KNOW THIS CHART FOR EXAM
Categorize by the Active/non replicating, maybe switch format of chart
HBV – Treatment and Prevention
Ongoing medical monitoring is essential
Antiviral therapy can suppress HBV replication
Vaccination is the most effective tool for HBV prevention
Contextual note: Vaccination leads to Anti-HBs production—mimicking recovery from infection without exposure to the virus.
HBV Vaccination Guidelines and Efficacy
National childhood immunization program began in 2005
↓ 98% in cases among <13 y/o
↓ 97% in cases among 12–19 y/o
Vaccination recommended for all at-risk adults, including:
Healthcare workers
Non-responders may receive a second vaccine series
Contextual note: Vaccination is the only route to Anti-HBs without prior infection—essential for prevention, especially in high-risk groups.
Hepatitis Viruses – Key Differences
Virus | Genome | Incubation | Transmission | Vaccine | Chronic? | Serology? |
|---|---|---|---|---|---|---|
A | RNA | 2–6 wk | Fecal–oral | Yes | No | Yes |
B | DNA | 8–26 wk | Parenteral, sexual | Yes | Yes | Yes |
C | RNA | 2–15 wk | Parenteral, sexual | No | Yes | Yes |
D | RNA | 2–8 wk | Parenteral, sexual | No | Yes | Yes |
E | RNA | 3–6 wk | Fecal–oral | No | Yes | Yes |
Contextual note: This table is useful for differentiating hepatitis types on exams, especially when comparing transmission, chronicity, and vaccine availability.
Want me to move on to the two timeline-based flashcards now?